Question
Question: How many following carbocation undergo re-arrangement- (a) $\text{CH}_3\text{CH}_2\text{CH}_2^+$ (b...
How many following carbocation undergo re-arrangement-
(a) CH3CH2CH2+ (b) (CH3)2C+HCHCH3
(c) (CH3)3C+HCHCH3 (d) (CH3CH2)3CCH2+ (g) CH2+−CH2−CH2CH3−CH2−O (h)
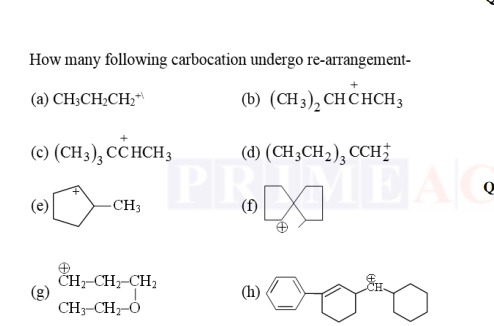
8
Solution
Carbocation rearrangement occurs when a less stable carbocation can convert into a more stable carbocation by a 1,2-shift of a hydrogen, alkyl group, or aryl group. Ring expansion/contraction can also occur to relieve strain or form a more stable ring system. The general order of carbocation stability is: resonance stabilized (especially by lone pairs) > tertiary > secondary > primary. Allylic and benzylic carbocations are also more stable due to resonance.
Let's analyze each carbocation:
(a) CH3CH2CH2+ (n-propyl carbocation) This is a primary (1°) carbocation. It can undergo a 1,2-hydride shift to form a more stable secondary (2°) carbocation: CH3CH2C+H21,2−H−shiftCH3C+HCH3 (isopropyl carbocation). Since a more stable carbocation is formed, it will undergo rearrangement.
(b) (CH3)2CHC+HCH3 (2-methylbutan-3-yl carbocation) This is a secondary (2°) carbocation. The adjacent carbon (C2, the one with two methyl groups) has a hydrogen. A 1,2-hydride shift can occur: CH3−CH(CH3)−C+H−CH31,2−H−shiftCH3−C+(CH3)−CH2−CH3 (2-methylbutan-2-yl carbocation). This new carbocation is tertiary (3°), which is more stable than secondary. So, it will undergo rearrangement.
(c) (CH3)3C−C+H−CH3 (2,2-dimethylbutan-3-yl carbocation) This is a secondary (2°) carbocation. The adjacent carbon (C2, the quaternary carbon with three methyl groups) does not have a hydrogen. However, a 1,2-methyl shift can occur: (CH3)3C−C+H−CH31,2−CH3−shift(CH3)2C+−CH(CH3)−CH3 (2,3-dimethylbutan-2-yl carbocation). This new carbocation is tertiary (3°), which is more stable than secondary. So, it will undergo rearrangement.
(d) (CH3CH2)3CCH2+ (3-ethyl-3-methylpentyl carbocation) This is a primary (1°) carbocation. The carbon adjacent to the carbocation is a quaternary carbon with three ethyl groups. A 1,2-ethyl shift can occur: (CH3CH2)3C−C+H21,2−CH2CH3−shift(CH3CH2)2C+−CH2CH3 (3,3-diethylpentan-3-yl carbocation). This new carbocation is tertiary (3°), which is more stable than primary. So, it will undergo rearrangement.
(e) 1-methylcyclopent-2-yl carbocation The carbocation is on a secondary carbon in a five-membered ring. An adjacent carbon (the one with the methyl group) is a secondary carbon with a hydrogen. A 1,2-hydride shift from E to A would move the positive charge to E: This new carbocation is tertiary (3°), which is more stable than secondary. So, it will undergo rearrangement.
(f) Spiro[4.4]nonan-1-yl carbocation The carbocation is on a secondary carbon in one of the five-membered rings. It is adjacent to a quaternary spiro carbon. A 1,2-alkyl shift (ring expansion) can occur where one of the bonds from the spiro carbon (E) to the ring (e.g., bond E-D) shifts to the carbocation carbon (A). This moves the positive charge to the spiro carbon (E) and expands the five-membered ring to a six-membered ring. The new carbocation on the spiro carbon (E) is tertiary (3°), and the ring strain is relieved by forming a six-membered ring. This makes it more stable. So, it will undergo rearrangement.
(g) CH2+−CH2−CH2−O−CH2−CH3 (3-ethoxypropyl carbocation) This is a primary (1°) carbocation. It can undergo a 1,2-hydride shift to form a secondary (2°) carbocation: CH2+−CH2−CH2−O−CH2−CH31,2−H−shiftCH3−C+H−CH2−O−CH2−CH3 (2-ethoxypropyl carbocation). This secondary carbocation can undergo another 1,2-hydride shift to place the positive charge on the carbon adjacent to the oxygen atom: CH3−C+H−CH2−O−CH2−CH31,2−H−shiftCH3−CH2−C+H−O−CH2−CH3 (1-ethoxypropyl carbocation). This final carbocation is highly stabilized by resonance due to the lone pair on the oxygen atom: CH3−CH2−CH=O+−CH2−CH3. This resonance stabilization makes it much more stable than the initial primary carbocation. So, it will undergo rearrangement.
(h) Phenyl-cyclohexenyl-cyclohexyl-methyl carbocation The carbocation is on a secondary carbon. This carbon is attached to a carbon of a cyclohexene ring and a carbon of a cyclohexane ring. The cyclohexene ring has a double bond conjugated with a phenyl group. The carbocation (E) is secondary. The adjacent carbon (D) in the cyclohexene ring is allylic to the double bond and also part of a conjugated system with the phenyl ring. A 1,2-hydride shift from D to E would move the positive charge to D: The new carbocation on D is a secondary carbocation that is both allylic and benzylic (due to extended conjugation with the phenyl group). This extended resonance stabilization makes it significantly more stable than the initial secondary carbocation. So, it will undergo rearrangement.
All 8 carbocations listed will undergo rearrangement to form more stable carbocations.
