Question
Question: How many equivalent hydrogens are there in each isomer of \({{C}_{3}}{{H}_{7}}Cl\) ?...
How many equivalent hydrogens are there in each isomer of C3H7Cl ?
Solution
The hydrogens which are attached to a carbon with the same chemical environment are called equivalent hydrogens. Neopentane has 12 hydrogens and all 12 hydrogens are the same due to the same chemical environment.
Complete step by step answer:
- In the questions it is asked how many equivalent hydrogens are there in each isomer of C3H7Cl .
- First we should know how many structural isomers are possible for C3H7Cl .
- The structural isomers for the given compounds are two and they are 1 – Chloropropane and 2 – Chloropropane.
- To know about the number of equivalent hydrogens in 1 – Chloropropane and 2 – Chloropropane we should know the structures of those compounds.
- The structures of 1 – Chloropropane and 2 – Chloropropane are as follows.
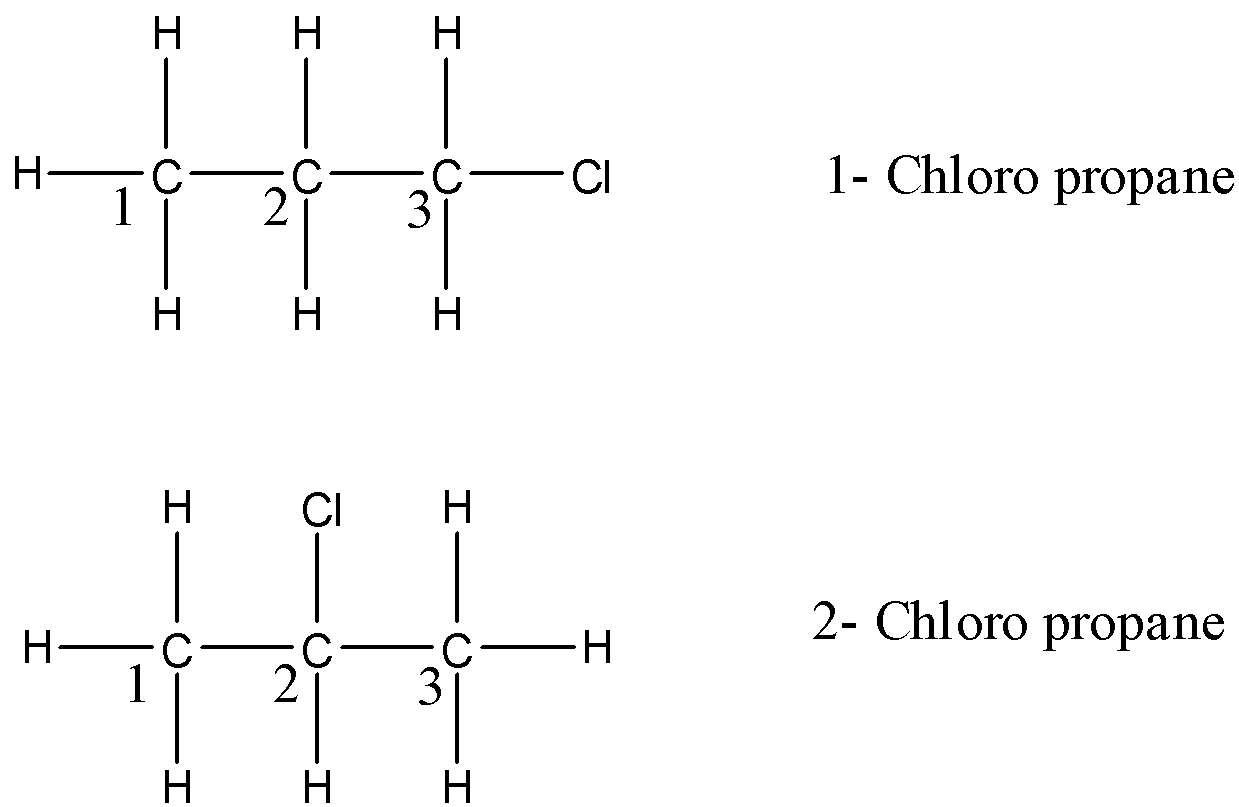
- From the above structures we can say that in 1 – Chloropropane there are three sets of equivalent protons present.
- In 1 – Chloropropane, on carbon-1 the three hydrogens have the same chemical environment and they are different from the hydrogen on carbon-2 because carbon-2 is nearer to chlorine which is present on carbon-1. So the hydrogens on carbon-1 are one set of equivalent protons.
- In 1 – Chloropropane, on carbon-2 the two hydrogens have the same chemical environment and they are different from the hydrogens on carbon-1 and carbon-3 because on carbon-1 there is a chlorine atom and on carbon-1 there is no chlorine. So, the hydrogens on carbon-2 are one set of equivalent hydrogens.
- In 1 – Chloropropane, on carbon-3 the two hydrogens have the same chemical environment and they are different from the hydrogens which are present on carbon-2 and carbon-3, because there is no chlorine on carbon-1 and carbon-3. So, the hydrogens on carbon-3 are one set of equivalent hydrogens.
- The same scenario repeats in the case of 2- chloro propane, it also has three sets of equivalent hydrogens.
- Therefore there are three sets of equivalent hydrogens present in each isomer of C3H7Cl .
Note: By using proton NMR spectroscopy we can easily find the equivalent hydrogens, in the proton NMR spectrum of 1- chloropropane there are three peaks at three different positions that confirm the presence of three sets of equivalent hydrogens.
