Question
Question: How many enolizable hydrogens are there in the following compound? 2-methylcyclohex2-en-1one A.2...
How many enolizable hydrogens are there in the following compound?
2-methylcyclohex2-en-1one
A.2
B.3
C.4
D.7
Solution
An enolizable ketone is one that has one or more alpha hydrogens in its molecule. The first carbon atom that binds to a functional group, such as a carbonyl, is referred to as the alpha carbon in organic molecules. The beta carbon is the second carbon atom, and the scheme continues to name them in alphabetical order using Greek characters.
Complete answer:
Keto–enol tautomerism is a chemical equilibrium between a keto form (a ketone or an aldehyde) and an enol in organic chemistry (an alcohol). Tautomers are said to exist between the keto and enol varieties. Since the interconversion of the two modes requires the passage of an alpha hydrogen atom as well as the reorganisation of bonding electrons, the isomerism is classified as tautomerism.
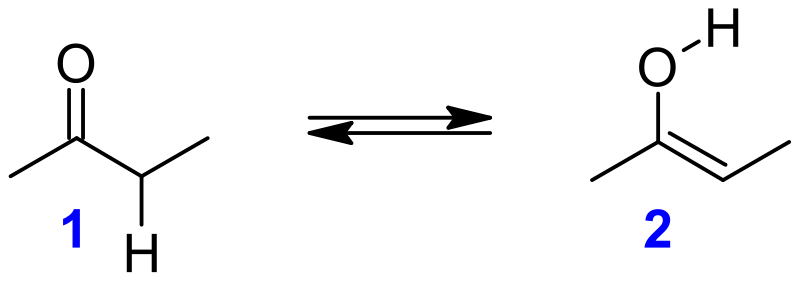
Alkenols, or enols, are a type of reactive structure or intermediate in organic chemistry that is described as an alkene (olefin) with a hydroxyl group attached to one end of the alkene double bond. The expressions enol and alkanol are portmanteau of "-ene"/"alkene" and the "-ol" suffix suggesting the hydroxyl group of alcohols, with the first term's terminal "-e" dropped. Deprotonation, or the elimination of a hydrogen adjacent (-) to the carbonyl ring, is often used in the production of enols. The consequence of not returning this proton at the end of the stepwise procedure is an anion known as an enolate.
2-methylcyclohex2-en-1one
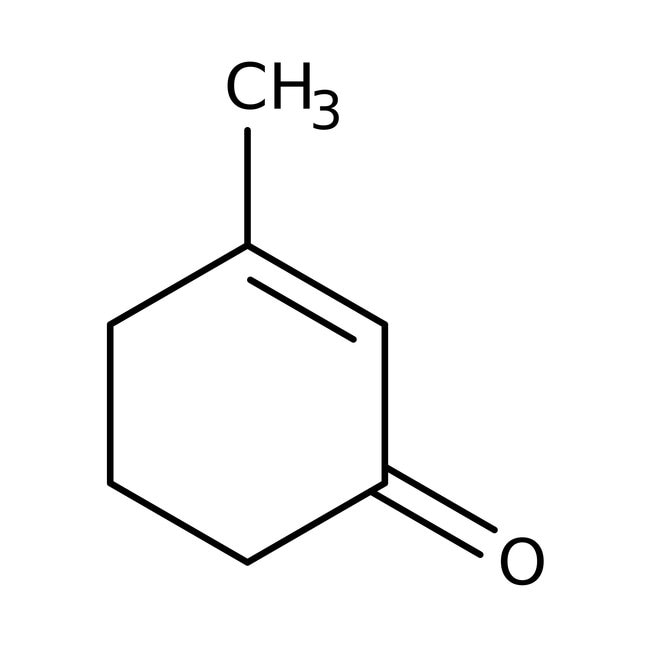
In the above structure, when "α" - Hydrogen in the molecule of an aldehyde or ketone is replaced as hydrogen ion, an enolate ion is formed.
α−H with respect to – C=O is 2
α−H with respect to double bond is 5
Total α−H observed = 2 + 5 = 7
Hence option D is correct.
Note:
The nomenclature may also be used to describe hydrogen atoms bound to carbon atoms. An alpha-hydrogen atom is a hydrogen atom attached to an alpha carbon atom; a hydrogen atom attached to a beta carbon atom is a beta hydrogen atom, and so on.
This naming standard does not conform to IUPAC nomenclature, which promotes carbons to be named by number rather than Greek letter, but it is still widely used, particularly for determining the relative position of carbon atoms to other functional groups.
