Question
Question: How many energetically equivalent resonance structures exist for oxalate dianion? 
A. 1
B. 2
C. 3
D. 4
Solution
Resonance structures are the hybrid of two or more structures obtained by a single compound. It differs in the position of electrons. Resonance mainly involves the delocalization of electrons within the molecules. Anion carries a negative charge and it symbolizes the excess of electrons present in the molecule. On the other hand, cation carries a positive charge and it symbolizes the deficiency of electrons present in the molecule.
Complete answer:
Oxalate dianion has the molecular formula C2O42− and it is obtained by the removal of two protons from oxalic acid. The possible resonance structures involved in oxalate dianion are,
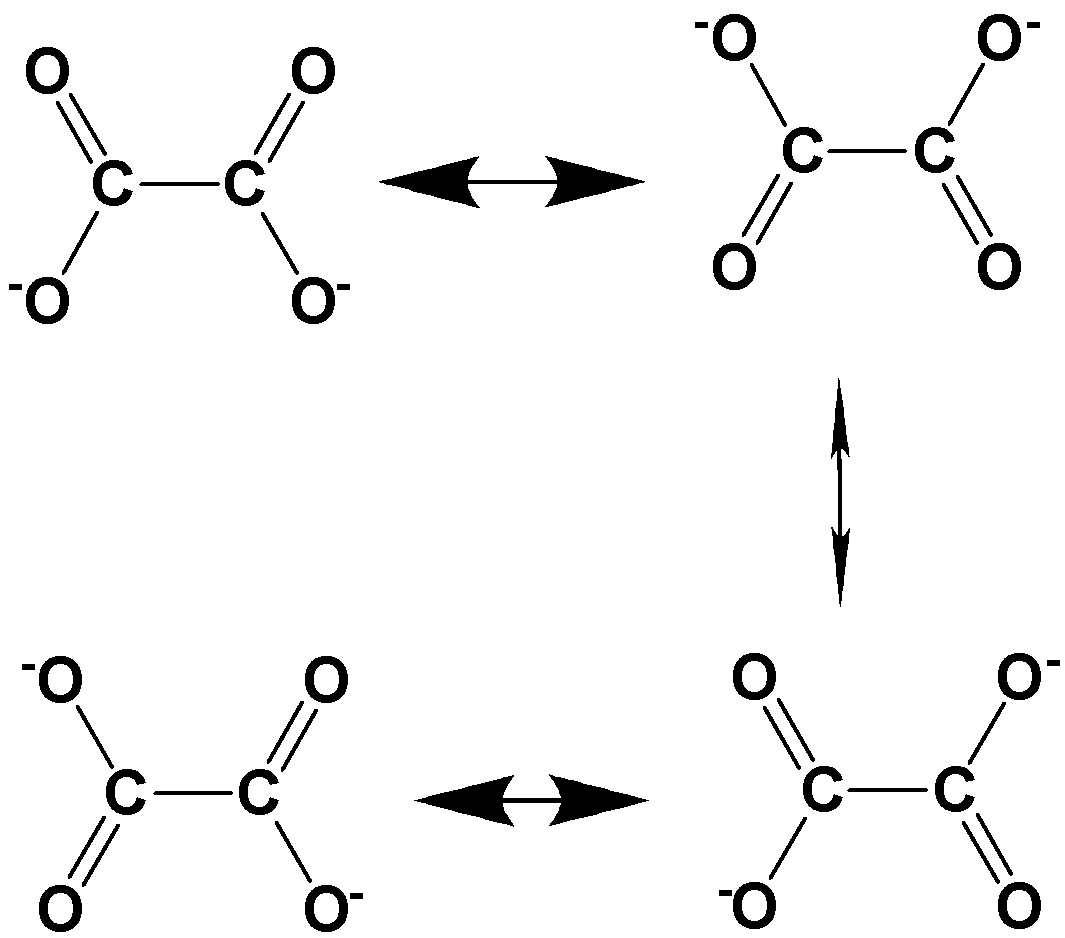
Thus, 4 energetically equivalent resonance structures exist for oxalate dianion.
Hence, option D is the correct option..
Note: It is already known that resonance is the delocalization of electrons within the molecules. Thus, the number of pi electrons present in the molecules must undergo resonating structures. This can be calculated by formula- P=6n+2−V
Where,
P-number of pi electrons
n-number of atoms present
V-total number of valence electrons in the molecule.
Here, n=6 since two carbons and four oxygen atoms are present in oxalate dianion.
A total number of valence electrons can be calculated by subtracting the charge of the given molecule to the sum of valence electrons present in the molecule.
V=(4+4+6+6+6+6)−(−2)
Since a carbon atom has four valence electrons and an oxygen atom has six valence electrons. The charge of oxalate dianion is negative and it has the value of −2
⇒V=32+2
⇒V=34
Thus, the total number of valence electrons can be calculated as,
P=6(6)+2−34
⇒P=36+2−34
⇒P=4
Four electrons in oxalate dianion are involved in the delocalization of electrons and therefore, the given oxalate dianion possesses four resonating structures.
