Question
Question: How many elements would be in the third period of the periodic table if the spin quantum number \({{...
How many elements would be in the third period of the periodic table if the spin quantum number ms could have the value −21, 0 and+21?
Solution
Think about all the quantum numbers along with the spin quantum number. Consider all the values the quantum number may take for the elements present in the third period of the periodic table.
Complete step by step solution:
First, we will consider the quantum numbers that can be assigned to all the orbitals for the elements in the third period of the periodic table.
We know that, if the value of principal quantum number (n) equals 3, then the azimuthal quantum numbers can have the values 0, 1 and 2. If the value of azimuthal quantum number (l) is equal to zero, then the magnetic quantum number (m) has only one value that is zero. The spin quantum number can traditionally only take the values +21,−21.
Similarly, if the value of azimuthal quantum number (l) is equal to 1, then the possible values for the magnetic quantum numbers are -1, 0 and +1. For each of these magnetic quantum numbers, 2−1 and 2+1 are the spin quantum numbers.
For azimuthal quantum number (l) is equal to 2, there are five different magnetic quantum numbers possible. They are -2, -1, 0, +1 and +2. For each of these magnetic quantum numbers, there are two possible values of spin quantum numbers, +21and−21.
Let us put all this information in a tabular form.
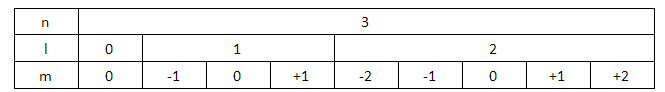
Here, the orbitals that have been assigned the azimuthal quantum number 2 are included in the 3d orbital, which will fill up after the 4s orbital. Thus, the elements that help in filling up those orbitals will not be included in the third period.
So, in the third period, the orbitals having only the first two azimuthal quantum numbers i.e. 0,1 will be considered. Each magnetic quantum orbital number consists of 2 electrons having spin quantum numbers +21,−21.
We know that each element has a different number of electrons than the other elements in the same period. Thus, each electron corresponds to 1 element. We can say that if 2 spin quantum numbers are available 8 elements will be present in the third period.
Now, as asked in the question, we will consider how many elements would exist in the third period if 3 electrons with different spins were allowed to be in one magnetic quantum orbital.
Considering azimuthal quantum numbers 0 and 1 containing 4 magnetic quantum orbitals in all. The number of electrons they can accommodate is 12 electrons.
Hence, 12 elements will be present in the third period if the spin quantum numbers of electrons were allowed to be +21,0 and −21
Note:
Remember that although the principal quantum number includes 3 azimuthal quantum numbers 0, 1, and 2. The azimuthal quantum number corresponds to the 3d orbital and thus should not be considered while calculating the number of elements in the third period.
