Question
Question: How many elements are there on the Periodic Table \(2020\) ?...
How many elements are there on the Periodic Table 2020 ?
Solution
Hint : Periodic table is also known as periodic table of elements. It is in a table form in which all the known (discovered) elements are arranged according to their atomic numbers, chemical properties and electronic configuration. The table has seven rows which are known as “periods” and eighteen columns which are known as “groups”.
Complete Step By Step Answer:
We know that, in a periodic table, all the known (discovered) elements have been arranged in the seven periods (rows) and eighteen (columns) of the table according to their atomic numbers, chemical properties and electronic configuration.
Now, we will see the period table:-
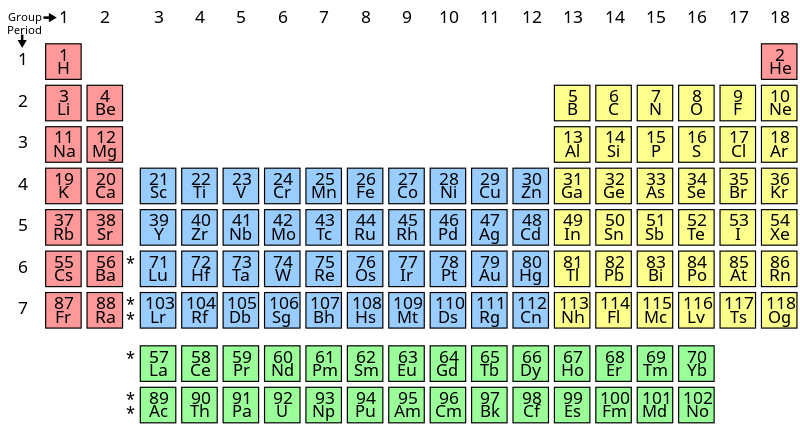
In the above periodic table, we can see that there are 118 known elements in 2020.
Additional Information:
The pink coloured elements in the group 1 and group 2 of the periodic table are known as s-block elements. They have general electronic configuration as ns1−2 .
The elements in the blue coloured boxes from group 3 to group 4 are known as d-block elements or transition metals. Their general electronic configuration is (n−1)d1−9ns1−2 .
The elements in the yellow coloured boxes from group 13 to group 18 are known as p-block elements or main-group elements. Their general electronic configuration is ns2np1−6 .
Group 18elements are known as noble gases as they are very stable and inert in nature.
The elements in the green coloured boxes at the downward side of the periodic table are known as f-block elements. Their general electronic configuration is (n−2)f(0−14)(n−1)d(0−10)ns2 . They are the members of group 3 in the periodic table. They are also known as inner- transition metals.
Note :
F- block (actinides and lanthanides) elements comprise of 28 elements and are always placed, with a notation, below the main body of the periodic table because actinides (Atomic numbers 58−71 ) and lanthanides ( atomic numbers 90−103 ) share similar chemical properties and cannot fit into the space inside the main body of periodic table.
