Question
Question: How many electrons should be removed from a coin of mass \(1.6g\), so that it may float in an electr...
How many electrons should be removed from a coin of mass 1.6g, so that it may float in an electric field of intensity 109NC−1 directed upward?
A) 9.8×107
B) 9.8×105
C) 9.8×103
D) 9.8×101
Solution
In the question, it’s given that the coin is floating. The coin will float only when the net force is zero or the forces are balanced i.e. the force due to the electric field is equal to the weight of the electron. From this, we can easily calculate the charge required to balance the electron and from that, we can calculate the amount of electron which will be removed.
Formulae used:
Ne=Ceq
Here Ne is the number of electrons, q is the total charge and Ce is the charge on one electron.
Complete step by step answer:
In the question, a coin of mass 1.6g is given. It is said that it is floating in the electric field of 109NC−1.
Let us draw the free body diagram of the coin.
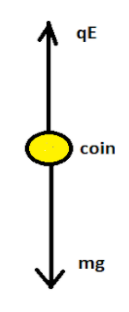
For the coin to float it should be in a balanced condition. The upward force acting on the coin should be equal to the downward force acting on the body. Hence
⇒qE=mg
Where q is the net charge on the coin, E is the electric field acting on the body, m is the mass of the coin and g is the acceleration due to gravity.
Let this be equation 1.
It’s given that,
m=1.6g=1.6×10−3kg
E=109NC−1
g=9.8ms−2
Putting the values of m, E and g in the equation 1, we get,
⇒q×109=1.6×10−3×9.8
∴q=1091.6×10−3×9.8
We know that the charge on one electron is 1.6×10−19C.
The number of electrons that is required to be removed,
⇒Ne=Ceq
Here Ne is the number of electrons, q is the total charge and Ce is the charge on one electron.
⇒Ne=Ceq
∴Ne=1.6×10−19C1091.6×10−3×9.8=9.8×107
So option (A) is the correct answer.
Note: Any charge when placed in an external electric field will experience an electric force. In this case the force experienced by all the electrons in the coin should balance the weight of the coin. We will not consider the electric force acting on the protons because this electric force acting on them is negligible as compared to the intranuclear forces inside the nucleus of the atoms. So, only the force on the electrons is considered.
