Question
Question: How many electrons in an atom may have the following quantum numbers: \(n=4\), \({{m}_{s}}=-\dfrac...
How many electrons in an atom may have the following quantum numbers:
n=4, ms=−21
A. 14
B. 16
C. 32
D. 20
Solution
Hint: We know that, there are a fixed number of electrons in each electron shell and the electrons attained in a fixed number are multiples of 2. Try to calculate the number of electrons using the direct formula of total number electrons in terms of n.
Complete step by step answer:
First, let us know about some terms related to the electron shell. The first is the principal quantum number which is denoted by the symbol ‘n’. They designate the principal electron shell of an atom. Its value is in n=1,2,3 and so on.
The second is orbital angular momentum which is represented by ‘l’ and it describes the shape of a given orbital and its value is equal to the total number of angular nodes in the orbital. If we have l=0, it means it has s-orbital and so on.
The third is spin quantum number which is denoted by ‘ms’ and this gives an idea about the direction in which the electron is spinning. It exists as +21 and −21 which means that an electron can spin in only two directions up and down. +21 is for spin up and −21 is for spin down.
Now, we know that the total number of electrons in n-shell can be calculated from the formula 2n2, where n denotes the number of shells.
We are given, n=4, ms=−21.
Therefore, the total number of electrons in n=4 shell will be 2×42=2×16=32. These electrons can be arranged as shown in the below figure:
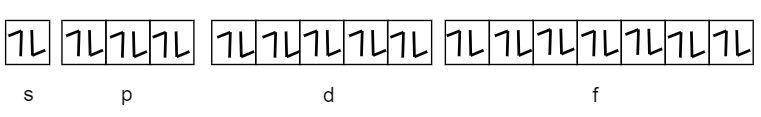
We are also given that the spin quantum number is −21, it means we have to consider the electrons with the negative spin only i.e. spin down.
Therefore, the total number of electrons for n=4 shells having −21 spin will be 16(as shown in the figure above).
Hence, the correct option is B.
Note: There can be confusion as to why we halved the number of electrons for negative spin. We did it because the number of total electrons we got by using the formula has an equal number of positive and negative spin.
