Question
Question: How many electrons are used in bonding the Lewis structure of \( {C_2}O_4^{2 - } \) (oxalate) ions?...
How many electrons are used in bonding the Lewis structure of C2O42− (oxalate) ions?
Solution
Each covalent and ionic bond is formed by the contribution of two electrons. So the total number of bonds has to be figured out using the correct lewis structure. Then the number of electrons involved in bonding can be calculated by multiplying the number of bonds by two.
Complete answer:
First, we need to figure out the lewis structure of the oxalate ion, which is –
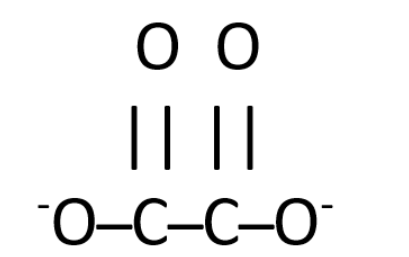
As the structure of oxalate ions suggests, there are a total of seven bonds present in one ion. As there are seven bonds so the number of electrons bonding will be twice of it, i.e. fourteen.
Hence, the correct answer is fourteen electrons are used in bonding the Lewis structure of C2O42− (oxalate) ions.
Note:
Oxalate ion is the conjugate base of oxalic acid, it reacts with metals and hydrogen to form compounds like sodium oxalate and oxalic acid. Calcium oxalate is the chief component of kidney stones. These are the product of incomplete oxidation of carbohydrates.
