Question
Question: How many electrons are in the valence shells of a) \(Be\) In \[BeC{{l}_{2}}\] b) \(B\) In \[BC{...
How many electrons are in the valence shells of
a) Be In BeCl2
b) B In BCl3
c) H In H2O
Solution
We know that a valence electron is an electron that is associated with an atom, and apparently that can participate in the formation of a chemical bond so we can form it in a single covalent bond, both atoms in the bond contribute one valence electron in order to form a shared pair. The key attribute is given by presence of valence electrons can determine the element's chemical properties and whether it may bond with other elements: For a main group element, we have to keep this in consideration that a valence electron can only be in the outermost electron shell.
Complete answer:
We know that Beryllium Be has 4 valence electrons in the compound BeCl2.
Be has 2 valence electrons of its own and shares 1 electrons with each of the two chlorine atoms. Electrons in Valence shells of Be in BeCl2 is given by:

b) Similarly Boron B has 6 valence electrons in the compound Boron Trichloride BCl3
B has 3 valence electrons of its own and shares 1 electrons with each of the three chlorine atoms. An electron in Valence shells of B in BCl3 is given by:
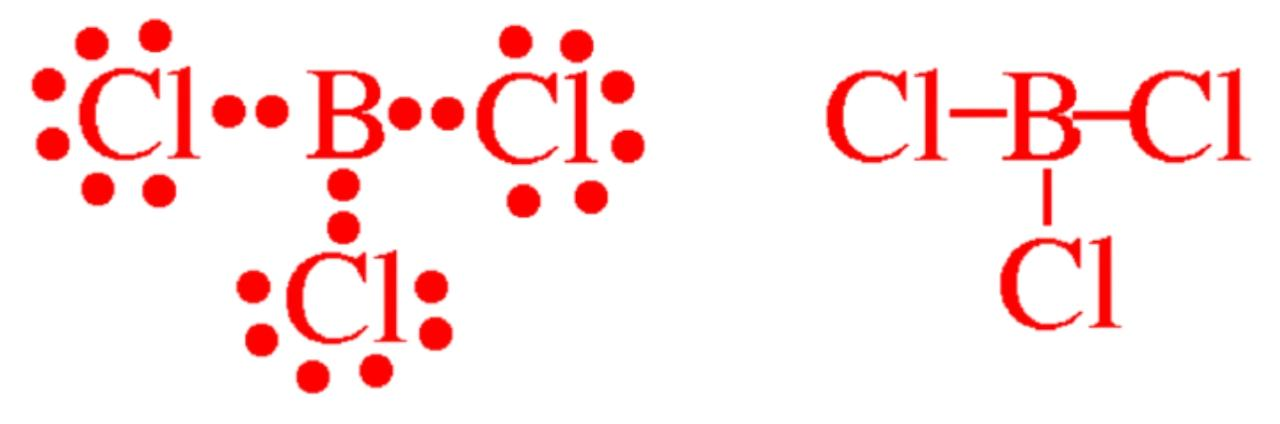
c) Likewise we have H in H2O where each H atom in H2O
H2O has 2 valence electrons. Each H atom has 1 valence electron of its own and shares 1 electron with the oxygen atom. An electron in Valence shells of H in H2O is given by:

Note: Also note that thiols are compounds that are structurally identical to alcohols except that they replace the oxygen atom in the hydroxyl group with a sulfur atom. Likewise, sulfides are structurally identical to ethers, but they replace the oxygen atom with a sulfur atom, as shown below.
