Question
Question: How many double bond equivalents (degrees of unsaturation) are in 2,2,3,3-tetramethyl-4-octyne?...
How many double bond equivalents (degrees of unsaturation) are in 2,2,3,3-tetramethyl-4-octyne?
Solution
The degree of unsaturation is a computation that calculates the total number of rings and π bonds in an organic molecule's chemical formula. In organic chemistry, a formula is used to aid in the drawing of chemical structures. It provides no information about the individual components, such as the number of rings, double bonds (one π bond each), or triple bonds (two π bonds each).
Complete answer:
An alkyne is an unsaturated hydrocarbon with at least one carbon—carbon triple bond in organic chemistry. With only one triple bond and no additional functional groups, the simplest acyclic alkynes form a homologous series with the generic chemical formula CnH2n−2.
The standard formula for computing the double bond equivalent (DBE) is:
DBE = C - 2H + 2N + 1
where C denotes the number of carbon atoms, H denotes the amount of hydrogen and halogen atoms, and N denotes the number of nitrogen atoms in the molecule if any exist.
The molecular formula of 2,2,3,3-tetramethyl-4-octyne can be given as C12H22
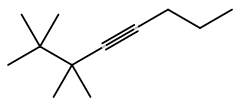
There dbe equals
DBE = 12 - 222 + 20 + 1 = 12 - 11 + 1 = 13 - 11 = 2
NMR, mass spectrometry, and IR spectroscopy, as well as qualitative examination, are used to verify the final structure. It works by comparing the current molecular formula to what may be a potential formula if the structure was saturated (no rings and just bonds) and all atoms had their normal valence.
Note:
Because joining two elements to form a ring or adding one extra bond in a structure reduces the need for two H's, the DBE (or IHD) for hydrocarbons tells us the number of rings and/or extra bonds in a non-saturated structure, which equals the number of hydrogen pairs required to make the structure saturated. For non-hydrocarbons, the elements in a pair can be any elements from the periodic table's lithium and fluorine families, although not necessarily all H's.
