Question
Question: How many donor atoms are present in EDTA?...
How many donor atoms are present in EDTA?
Solution
We know that, the full name of EDTA is ethylenediaminetetraacetic acid and its molecular formula is C10H16N2O8. It has pH value between −0.8to12. EDTA is versatile in nature. It can be used in the medical area and industrial area. It behaves as an acid, aminopolycarboxylic acid. It shows many side effects.
Complete step by step solution
Now, let’s discuss EDTA (ethylenediaminetetraacetic acid) in detail.
As we know that EDTA is a chelating agent, which is used in the separation of dye and other substances from heavy metals.it is a type of compound which can be used in household and industrial areas. In European inland, it has the highest concentration that is why it is named as an anthropogenic compound. Studies have detected poor biodegradability in the natural ecosystem. The structure of EDTA is shown below.
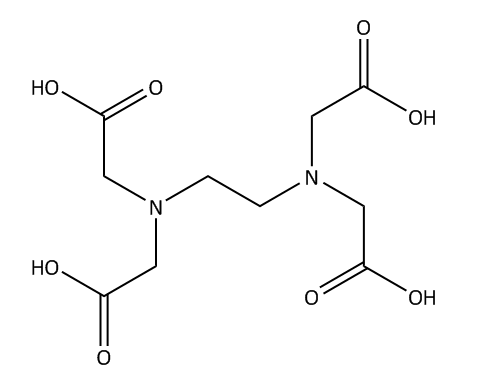
It is a hexadentate ligand, which means it has 6 lone pair of electrons that participate in coordination bonding. When a metal reacts with one molecule of EDTA, it can form 6 valent coordination complex. Metal ions have 4bonds on oxygen atoms that are negatively charged and 2 bonds to a single electron pair on the nitrogen atom.
**Therefore, EDTA has six donor atoms.
Notes: **
EDTA is used as an anticoagulant for blood as it forms chelate with the calcium ions present in the blood responsible for the coagulation of blood. It is concluded that EDTA acts in the environment as a persistent substance and that its contribution to bioavailability and remobilization of heavy metals.
