Question
Question: How many different acyclic isomers of \({{\text{C}}_{\text{6}}}{{\text{H}}_{{\text{12}}}}\) on hydro...
How many different acyclic isomers of C6H12 on hydrogenation with H2/Ni gives the same 3-methyl pentane?
Solution
Compounds that have identical chemical formula but different structures are known as isomers of each other. Alkanes are obtained by the hydrogenation of alkenes.
Complete step by step answer:
The product obtained is 3-methyl pentane. In 3-methyl pentane, the parent compound is pentane which suggests that 3-methyl pentane is an alkane.
The structure of 3-methyl pentane is,
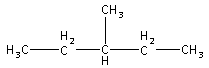
3-methyl pentane is obtained by hydrogenation of alkenes with H2 in presence of nickel as a catalyst. All the alkenes are acyclic isomers of C6H12.
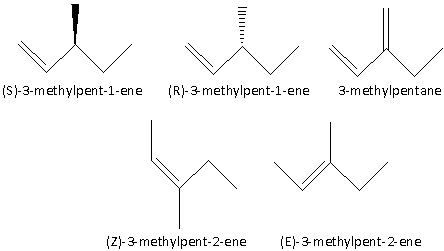
The alkenes which are acyclic isomers of C6H12 are as follows:
1.(S)-3-methylpent-1-ene
2.(R)-3-methylpent-1-ene
3.3-methylpentane
4.(Z)-3-methylpent-2-ene
5.(E)-3-methylpent-2-ene
The structures of the acyclic isomers are as follows:
Note: The hydrogenation of alkenes with H2 in presence of nickel as a catalyst gives alkanes. The H2 gets added across the carbon-carbon double bond resulting in formation of an alkane. Thus, hydrogenation of an alkene is an addition reaction.
The hydrogenation of alkynes with H2 in presence of nickel as a catalyst gives alkenes. The H2 gets added across the carbon-carbon triple bond resulting in formation of an alkene. Thus, hydrogenation of an alkyne is an addition reaction.
