Question
Question: How many diastereomers are there of glucose?...
How many diastereomers are there of glucose?
Solution
The diastereomers are not mirror images of each other and are non-superimposable. The molecular formula of glucose is C6H12O6. The maximum number of diastereomers present in a molecule is 2n−2.
Complete step by step answer:
Diastereomers are the compounds which are not mirror images of each other and are non-superimposable in other hand the enantiomers are the mirror image of each other and are non-superimposable. The diastereomers and the enantiomers are commonly called stereoisomers which is the class of isomerism.
When two molecules are stereoisomers which means they possess the same molecular formula, same connectivity but different arrangement of atoms but are not enantiomers then they are considered as diastereomers.
As we know that the maximum optical isomerism can be 2n where the n represents the number of chiral centers. The chiral centers are defined as the center where the atom is bonded to four different atoms or groups.
The glucose is the chemical compound with molecular formula C6H12O6. The two enantiomers of glucose are D-glucose and L-Glucose.
The structure of glucose is shown below.
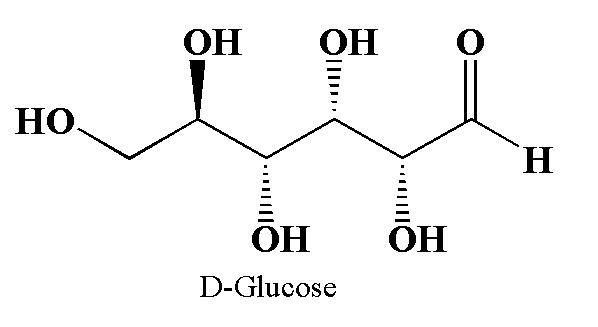
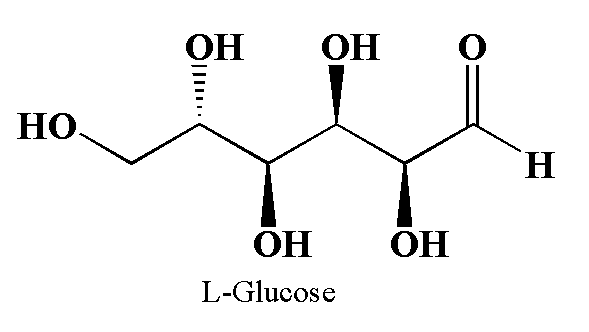
The above diagram is drawn using Chemdraw.
In D-glucose 4 stereocenters are present. So the number of optical isomers will be (2)4= 16 which includes D-glucose.
In L-Glucose all the stereocenters are inverted as compared to the D-glucose.
As, the maximum number of diastereomers is 2n−2, so the number of diastereoisomer is glucose is
⇒16−2=14
Therefore, 14 diastereomers are there of glucose.
Note: Diastereomers which differ from each other by one stereocenter out of two or more stereocenters are called epimers. D-galactose is one of the 14 diastereoisomers of glucose is the epimer along with D- glucose.
