Question
Question: How many cyclopentane structures (excluding stereoisomers) are possible for\({{C}_{7}}{{H}_{14}}\) ....
How many cyclopentane structures (excluding stereoisomers) are possible forC7H14 .
Solution
Cyclopentane is an organic chemical containing five carbon atoms present in a cyclic ring. Generally cyclic organic compounds up to five membered rings are less stable in nature when compared to aliphatic hydrocarbons of the same length.
Complete step-by-step answer: - In the question it is asked to draw the structures of cyclopentane that are possible with the molecular formula of C7H14 by excluding the stereoisomers.
- In the given molecular formula there are seven carbon atoms and fourteen hydrogen atoms are present.
- The basic structure of cyclopentane is as follows.
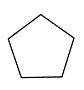
- Five carbon atoms are going to attached end to end each other.
- Stereoisomers are the isomers that have the same molecular formula and same sequence of bonds but they differ in three dimensional orientations.
- The possible cyclopentane structures with the molecular formula are as follows.
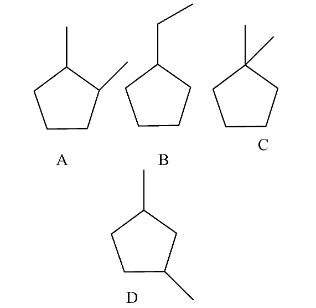
- There are only four possible cyclopentane structures by excluding the stereoisomers with the molecular formula of C7H14.
- Structure D and B can form two stereoisomers.
- Structure C and A can form one more stereoisomer with the same molecular formula C7H14.
- But we need the possible isomers by excluding stereoisomers.
- Therefore the possible cyclopentane structures are four only with molecular formula C7H14 by excluding stereoisomers.
Note: Stereoisomers are also called as spatial isomers; they have the same molecular formula but different three dimensional arrangements of atoms. Stereoisomers are mirror images and are optically active in nature.
