Question
Question: How many covalent bonds can a carbon atom form with neighboring atoms?...
How many covalent bonds can a carbon atom form with neighboring atoms?
Solution
We need to know what are covalent bonds and what are the factors which determine the number of covalent bonds an element can form with other atoms. The attractive force which holds various constituents such as atoms and ions together in different chemical species is called a chemical bond. A covalent bond is a chemical bond that is formed by the sharing of electron pairs between atoms. Carbon atoms belong to group 4 of the periodic table with atomic number 6 whose covalency we are to study.
Complete step by step answer:
We must know that a strict rule is followed by atoms during covalent bonding which is the octet rule. According to this rule, main group elements tend to bond in such a way, either by losing, gaining or sharing electrons such that each atom has 8 electrons in its outermost (valence shell). This craving of elements to satisfy its octet is the main reason for the chemical bonding. The valence electrons can be determined by writing the electronic configuration of the element.
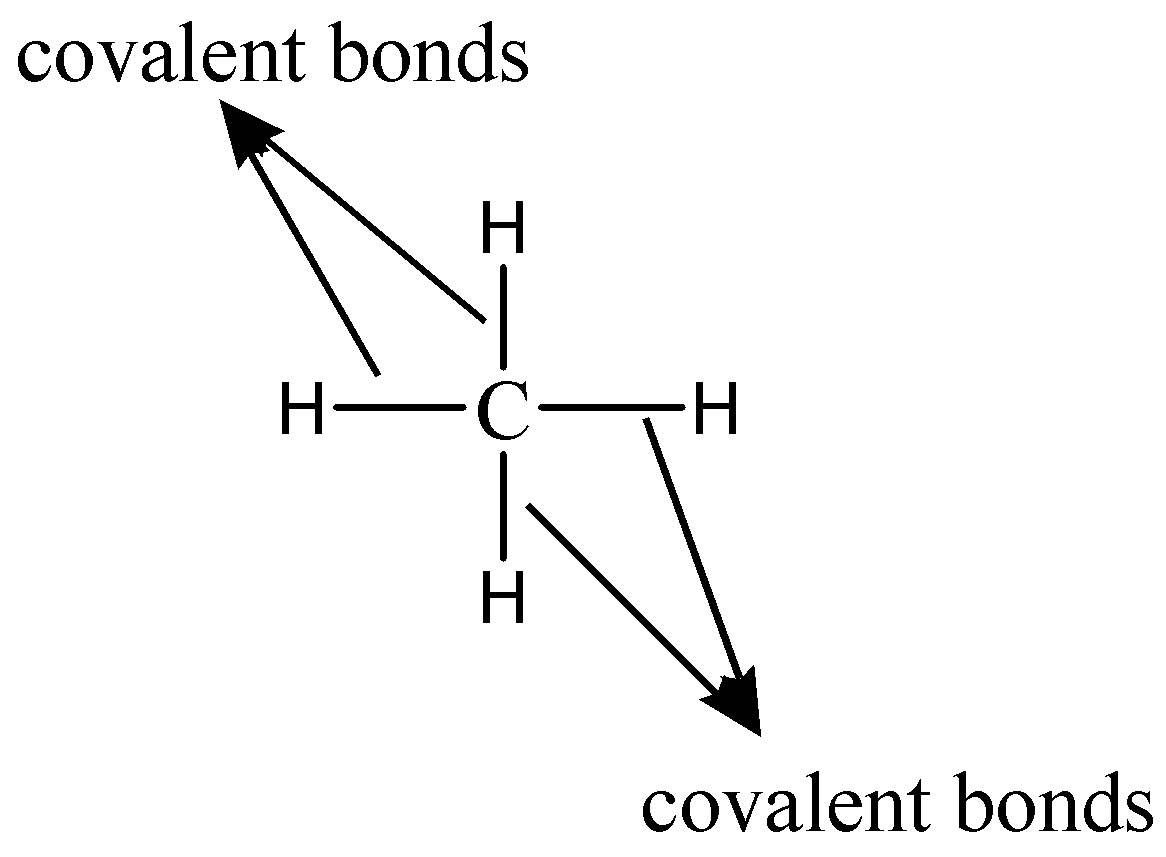
As we know that the electronic configuration of Carbon whose chemical symbol is C and atomic number is 6 has the electronic configuration 1s22s22p2 . Hence, it is clear that it has 4 electrons in its valence shell. These 4 electrons can be involved in covalent chemical bonding. Therefore, it can form four covalent bonds with other atoms. The simplest example to show the four covalent bonds which a carbon atom can form with neighboring atoms is methane (CH4). The bonding is shown as follows:
Note:
It must be noted that carbon is called a tetravalent element means it can form four bonds with other atoms. Any of the hydrogen atoms as given in the methane molecule can be replaced with another carbon atom covalently bonded to the first carbon atom. In this way, long and branching chains of carbon compounds can be made which is responsible for the diversity of almost all biological macromolecules.
