Question
Question: How many constitutional isomers does\({{C}_{3}}{{H}_{8}}O\) have?...
How many constitutional isomers doesC3H8O have?
Solution
Isomers are compounds having the same molecular formula but different structural formula. Isomers can be of various types like, geometrical, constitutional, stereoisomer etc. constitutional isomers contain different positions of the alkyl groups, and these are same as structural isomers.
Complete answer:
Isomers are defined as the compounds having the same chemical formula but having different structural representations. There are various types of isomers; among them are the constitutional isomers that have the same molecular formula but the position of the functional groups or the alkyl group changes.
We have been given a compound that has 3 carbon, 8 hydrogen and 1 oxygen, asC3H8O, as the general formula of alcohols suggests thatCnH2n+1OH a 3 carbon alcohol, or propanol will haveC3H7OH that isC3H8O. So, the compound can be known as an alcohol.
For alcohol, propanol can exist in 2 constitutional isomers that are:
1-propanol whose structure has OH functional group at 1st carbon as 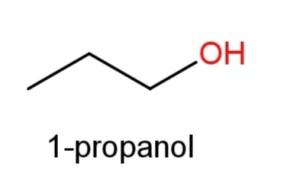 and
and
2-propanol whose structure has OH group at the second carbon atom as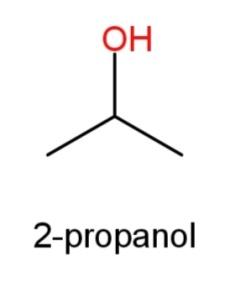
Now, a 3rd isomer is also possible when we do not consider OH functional group, and consider an ether as a functional group that has formula R – O – R, where R is the alkyl group, so with 3 carbon it can be:
Ethyl methyl ether whose structure has O in the center and an ethyl and methyl group at the ends as,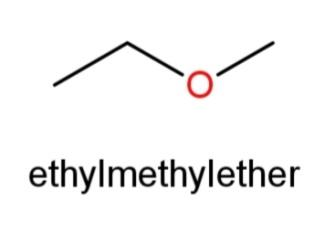
Hence, 3 constitutional isomers ofC3H8Oare possible that are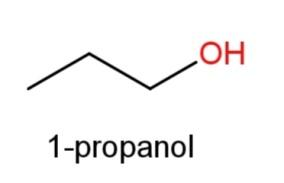 ,
,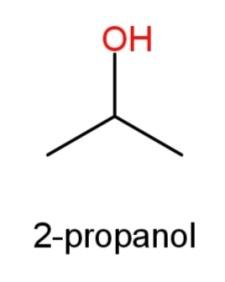 ’
’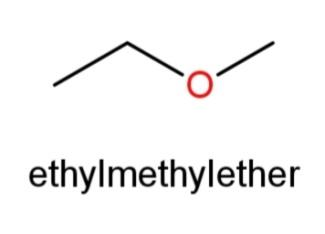 .
.
Note:
The constitutional isomers can be of various types, here we have made functional isomers in which the position of functional groups changes the structures. When the carbon skeleton is rearranged then it is called skeletal isomers, when the alkyl groups are made as straight chains or taken as branches then they are termed as positional isomers.
