Question
Question: How many constitutional isomers does \({{C}_{3}}{{H}_{8}}O\) have?...
How many constitutional isomers does C3H8O have?
Solution
Constitutional isomers are those isomers which have the same number of atoms of each of the elements but differ in the presence of bonds between them that is which is having the distinct bond between them.
Complete step by step answer:
We are familiar with the concepts of organic chemistry which tells us about the types of isomers that can be formed by one molecule and also the variation in their structures or geometries.
Now, we shall see the detailed study about the isomers and its types which also include the constitutional isomers.
- Isomers are the molecules with the identical molecular formula but have the distinct arrangement of atoms in space.
- There are many types of isomers among which constitutional isomer or metamer is one among them which is defined as isomers which have the same number of atoms of each of the elements but are having the distinct bond between them.
- In the given formula, C3H8O we can write this in the general formula as CnH2n−2O, where in if we put n = 3 we get the same formula
- For this type of compound we get two types of functional group that is monohydric alcohol or another possibility is aliphatic ether.
a) The only ether which is possible with this formula will be methoxy ethane that is CH3−O−CH2−CH3
b) The monohydric alcohol can be of two forms one is pentan-1-ol which is as shown below,
CH3−CH2−CH2−OH
The other possibility is the pentan-2-ol which is shown below:
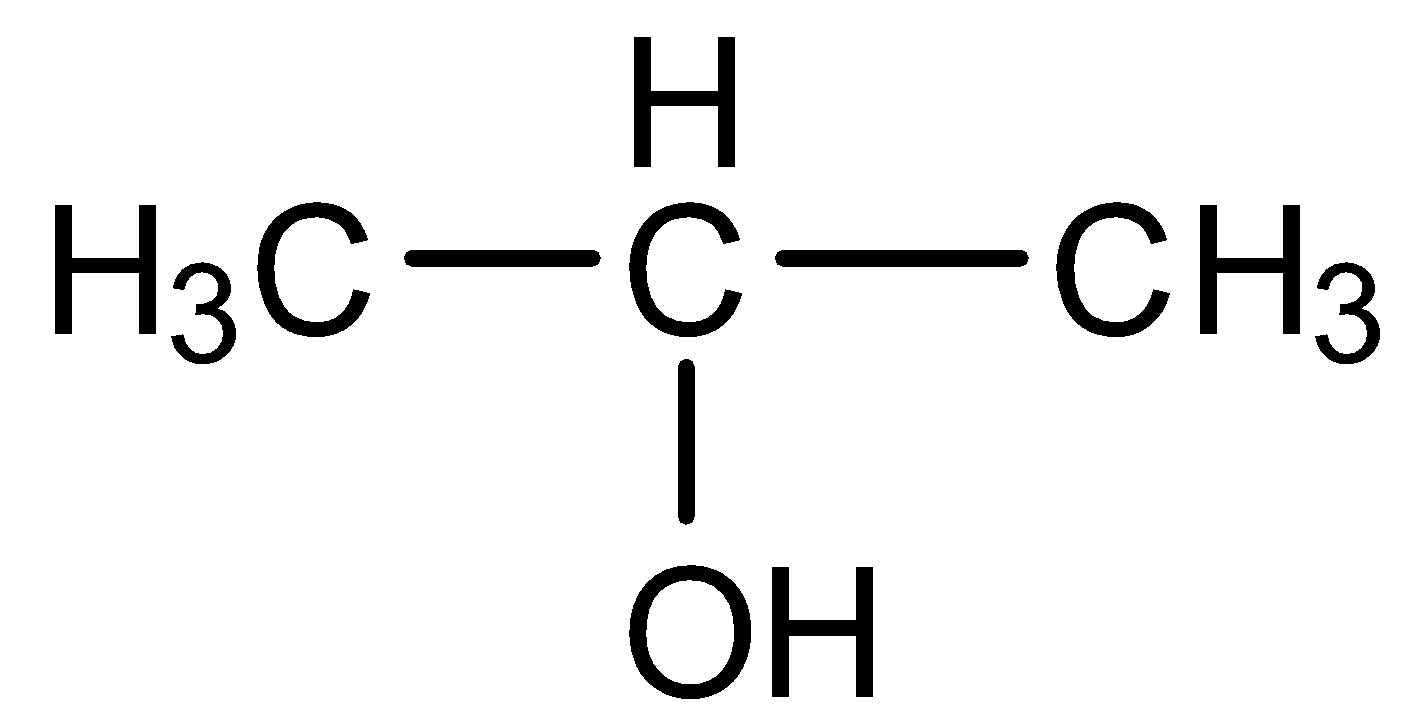
Thus, the correct answer is three constitutional isomers are possible for the molecular formula C3H8O
Note: Constitutional isomers were also called as metamers formerly which has the same meaning and this concept also applies to the polyatomic ions with the same total charge and the example which can be cited is the cyanate ion.
