Question
Question: How many constitutional isomers are possible for \({{\text{C}}_{\text{4}}}{{\text{H}}_{\text{9}}}\te...
How many constitutional isomers are possible for C4H9Br?
Solution
Constitutional isomers are those isomers which have the same molecular formula but differ in the connectivity between the atoms.
The given compound is an alkane.
Complete step by step answer:
So in the question, it is asked how many constitutional isomer structures are possible for the given hydrocarbon which is C4H9Br.
- From the time we are studying organic chemistry we are studying about various hydrocarbons. The hydrocarbons are those chemical compounds which are formed by hydrogen and carbon.There are mainly four types of hydrocarbons with each having the general formulae of its own.
- The four types of hydrocarbons are –alkanes, alkenes, alkynes and aromatic hydrocarbons.
The given compound C4H9Br is an alkane, since it satisfies the general formula of alkane which is CnH2n+2, one H atom is replaced by Br.
So we now know that the structure does not have a double bond or triple bond. In the formulae given, there are four carbons hence the parent chain is butane.
- Now let’s see what are isomers and what is constitutional isomers?
Isomers are those molecules which have similar molecular formula but differ either in their structural arrangement or spatial arrangement.
- The constitutional isomers are those isomers which have the same molecular formula but have different structures.The constitutional isomers are the type of structural isomers and they differ in the connectivity of the atoms.
So now let’s see how many constitutional isomers are possible for the given compound.
- First let’s write the first degree or primary carbon chain ie the normal straight chain which has four carbons and the bromine attached to the first carbon.And the structure is:
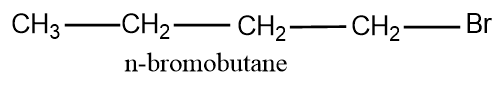
Now let’s change the position of Br and arrange it in the second carbon and the structure obtained is:
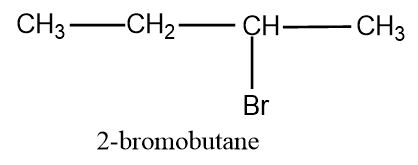
The above given structure is a secondary structure and another secondary structure is also possible which is:
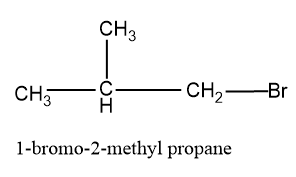
In this structure the methyl group is shifted to the second carbon and the main chain taken here is propane,since it is the longest unbranched chain.
The tertiary structure for the given compound is:
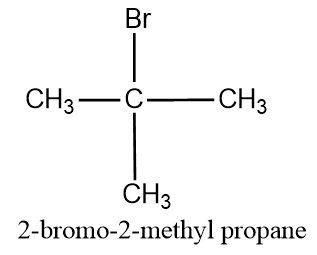
- Here in the structure the Br atom and methyl group is attached to the same atom, the second atom,which yields a tertiary structure.
So these are the possible four constitutional isomers for C4H9Br. All these molecules have the same number of carbon atoms, H atoms and Br the only difference is in their structure due to its difference in the connectivity of atoms.
Note: The general formula for alkenes are :CnH2n
The general formula for alkyne are: CnH2n−2
- If the difference in isomeric structures is due to the arrangement of atoms in space, then that type of isomerism is called stereoisomers and it is of two types : Geometrical isomers and optical isomers.
- The structural isomers are also divided into many: chain isomers -in which the length of the carbon main chain differs ,position isomers- in which the position of the substituents or multiple bond varies but molecular formulae remains the same, functional isomers- in which the structure have different functional groups but the molecular formulae will be same.
