Question
Question: How many compounds can be obtained in yield by Wurtz reaction? 
(a)- 6
(b)- 3
(c)- 4
(d)- 5
Solution
Wurtz reaction is a preparation method in which we can prepare symmetrical alkanes, the yield of a symmetrical alkane is good, but unsymmetrical alkanes are not easily prepared in this reaction. In this process, two moles of alkyl halide are treated with sodium and we get alkane.
Complete step-by-step answer: Wurtz reaction is a preparation method in which we can prepare symmetrical alkanes, the yield of a symmetrical alkane is good, but unsymmetrical alkanes are not easily prepared in this reaction. In this process, two moles of alkyl halide are treated with sodium and we get alkane.
The general reaction for this method will be:
2R−X+2Na→R−R+2NaX
Let us study all the options given above:
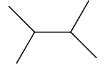
This compound will be made from a secondary halide having the formula CH3−CH(CH3)−X. The reaction is given below:
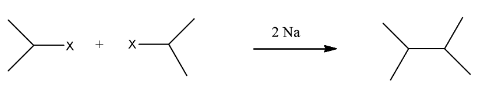
This compound is a symmetrical alkane, hence it will have a good yield.
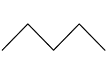
This compound will be made from primary halides having formula CH3−CH2−CH2−X and CH3−CH2−X. The reaction is given below:
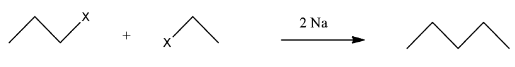
This compound is an unsymmetrical alkane, hence it will not have a good yield.
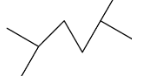
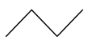
This compound will be made from primary halide having formula CH3−CH(CH3)−CH2−X. The reaction is given below:
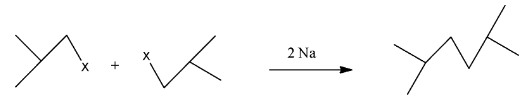
This compound is a symmetrical alkane, hence it will have a good yield.
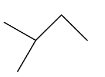
This compound will be made from primary and secondary halides having formula CH3−CH(CH3)−X and CH3−CH2−X. The reaction is given below:
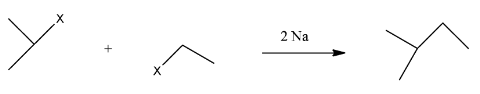
This compound is an unsymmetrical alkane, hence it will not have a good yield.
This compound will be made from a primary halide having the formula CH3−CH2−X. The reaction is given below:
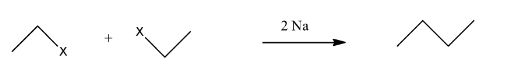
This compound is a symmetrical alkane, hence it will have a good yield.
CH4 has only one carbon atom, hence it cannot be prepared from the Wurtz reaction.
Therefore, the correct answer is an option (b)- 3.
Note: The Wurtz reaction follows the bimolecular Nucleophilic substitution reaction, i.e., SN2 mechanism. The smallest alkane that can be prepared from this method will be ethane.
