Question
Question: How many compounds among the following compounds show inductive, mesomeric as well as hyperconjugati...
How many compounds among the following compounds show inductive, mesomeric as well as hyperconjugation effects?
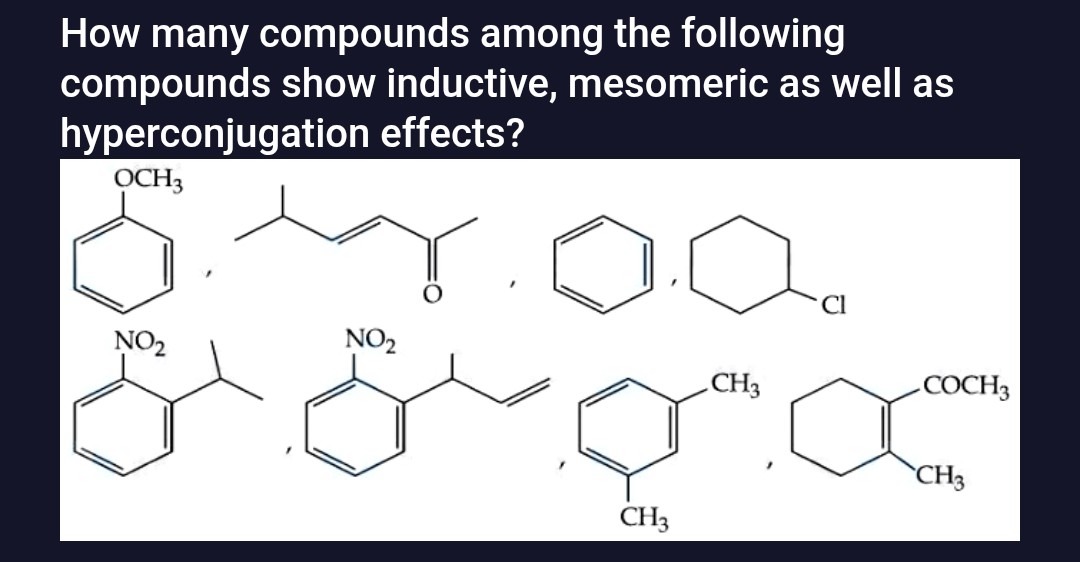
Methoxybenzene (Anisole)
3-Methylpent-3-en-2-one
Benzene
Chlorocyclohexane
2-Isopropyl-1-nitrobenzene
2-Allyl-1-nitrobenzene
3-Methylanisole (m-Methylanisole)
1-Acetyl-2,6-dimethylcyclohex-1-ene
6
Solution
Here's an analysis of each compound to determine if it exhibits inductive, mesomeric, and hyperconjugation effects:
1. Methoxybenzene (Anisole)
- Inductive Effect (I): Present due to the electronegativity difference between C and O in the C-O bond, and C-H bonds. Oxygen is electronegative (-I effect from O, +I effect from CH₃).
- Mesomeric Effect (M): Present. The lone pairs on the oxygen atom of the -OCH₃ group can be delocalized into the benzene ring, exhibiting a +M effect.
- Hyperconjugation (H): Present. The C-H bonds of the methyl group are adjacent to the oxygen atom, which is attached to the aromatic ring. These C-H sigma bonds can overlap with the adjacent C-O sigma bond or the pi system of the benzene ring, contributing to hyperconjugation.
- Exhibits all three effects.
2. 3-Methylpent-3-en-2-one CH3−CH=C(CH3)−C(=O)−CH3
- Inductive Effect (I): Present due to C-C, C-H, C=C, and C=O bonds. Methyl groups are +I, carbonyl carbon is -I.
- Mesomeric Effect (M): Present. The C=C double bond is conjugated with the C=O double bond, allowing for resonance (electron delocalization). The C=O group exhibits a -M effect.
- Hyperconjugation (H): Present. There are alpha-hydrogens on the methyl group attached to the double bond (CH₃-CH=) and on the methyl group of the acetyl moiety (-C(=O)-CH₃). These hydrogens are adjacent to sp² hybridized carbons (double bond and carbonyl carbon attached to the double bond), allowing for hyperconjugation.
- Exhibits all three effects.
3. Benzene
- Inductive Effect (I): Present due to C-C and C-H sigma bonds.
- Mesomeric Effect (M): Present. The delocalization of pi electrons in the aromatic ring is a mesomeric effect.
- Hyperconjugation (H): Not present. Benzene itself does not have alkyl substituents with alpha-hydrogens adjacent to a pi system or charged center.
- Does not exhibit all three effects.
4. Chlorocyclohexane
- Inductive Effect (I): Present due to the electronegativity difference between C and Cl in the C-Cl bond (-I effect of Cl).
- Mesomeric Effect (M): Not present. There is no pi system or lone pair conjugation.
- Hyperconjugation (H): Not present. There are no double bonds, aromatic rings, carbocations, or radicals.
- Does not exhibit all three effects.
5. 2-Isopropyl-1-nitrobenzene
- Inductive Effect (I): Present due to C-C, C-H bonds in the ring and isopropyl group. The nitro group (-NO₂) exhibits a strong -I effect. The isopropyl group exhibits a +I effect.
- Mesomeric Effect (M): Present. The nitro group (-NO₂) is directly attached to the benzene ring and exerts a strong -M effect.
- Hyperconjugation (H): Present. The isopropyl group [-CH(CH₃)₂] has methyl groups whose C-H bonds are alpha to the carbon attached to the benzene ring. These alpha-hydrogens can participate in hyperconjugation with the pi system of the benzene ring.
- Exhibits all three effects.
6. 2-Allyl-1-nitrobenzene o-NO2-C6H4-CH2-CH=CH2
- Inductive Effect (I): Present due to C-C, C-H bonds in the ring and allyl group. The nitro group (-NO₂) is -I. The allyl group has C=C, which influences electron distribution.
- Mesomeric Effect (M): Present. The nitro group (-NO₂) is directly attached to the benzene ring and exerts a strong -M effect.
- Hyperconjugation (H): Present. The allyl group (-CH₂-CH=CH₂) has alpha-hydrogens on the methylene group (-CH₂-) directly attached to the ring, and also alpha-hydrogens on the vinylic methylene group (-CH=CH₂). These contribute to hyperconjugation.
- Exhibits all three effects.
7. 3-Methylanisole (m-Methylanisole)
- Inductive Effect (I): Present due to C-C, C-H bonds, and the C-O bond. -OCH₃ group is -I (due to O) and +I (due to CH₃). CH₃ group is +I.
- Mesomeric Effect (M): Present. The lone pairs on the oxygen atom of the -OCH₃ group can be delocalized into the benzene ring, exhibiting a +M effect.
- Hyperconjugation (H): Present. The C-H bonds of the methyl group (-CH₃) attached to the benzene ring can participate in hyperconjugation with the pi system of the ring.
- Exhibits all three effects.
8. 1-Acetyl-2,6-dimethylcyclohex-1-ene (Assuming the structure is as depicted: a cyclohexene ring with a double bond, one carbon of the double bond has -COCH₃, the other has -CH₃, and a third -CH₃ is on a saturated carbon of the ring, likely at position 6 relative to the double bond).
- Inductive Effect (I): Present due to C-C, C-H, C=C, and C=O bonds. Methyl groups are +I, carbonyl carbon is -I.
- Mesomeric Effect (M): Present. The C=C double bond is conjugated with the C=O double bond of the acetyl group, allowing for resonance. The C=O group exhibits a -M effect.
- Hyperconjugation (H): Present. There are alpha-hydrogens on the methyl group attached to the double bond (at C2) and on the methyl group of the acetyl moiety (-COCH₃). These hydrogens are adjacent to sp² hybridized carbons and contribute to hyperconjugation. The methyl group at C6 also has alpha-hydrogens attached to a saturated carbon, which can contribute to hyperconjugation, although usually less significantly than those directly adjacent to pi systems. However, for the presence of the effect, it is considered.
- Exhibits all three effects.
Summary: The compounds that show inductive, mesomeric, as well as hyperconjugation effects are:
- Methoxybenzene (Anisole)
- 3-Methylpent-3-en-2-one
- 2-Isopropyl-1-nitrobenzene
- 2-Allyl-1-nitrobenzene
- 3-Methylanisole
- 1-Acetyl-2,6-dimethylcyclohex-1-ene
Therefore, there are 6 such compounds.
