Question
Question: How many chloride ions are surrounding a sodium ion in a sodium chloride crystal? (A) 4 (B) 8 ...
How many chloride ions are surrounding a sodium ion in a sodium chloride crystal?
(A) 4
(B) 8
(C) 6
(D) 12
Solution
Sodium chloride has a FCC unit cell such that the chloride ions are present at the corners and the face centres of the unit cell and the sodium ions are present at the edge centres of the unit cell and the body centre of the unit cell which are essentially the octahedral voids.
Complete step by step answer:
For solving this question, we need to understand the structure of the face centred cubic unit cell.
The diagram of Face centred cubic unit cell is given below:
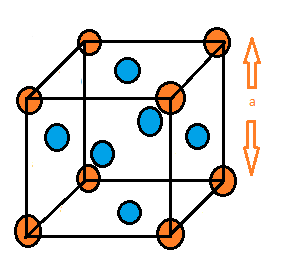
In the diagram above, the atoms present at the corners do not touch each other. But the atoms present at the face centres touch the atoms present at the corners of that face. Here the atoms are present at the corners as well as at the face centres. The atoms present at the corners are shared among 8 unit cells while the atoms present at face centres are shared among 2 unit cells. Therefore the total number of atoms per unit cell is = 8×81+6×21=4
Let us calculate the packing efficiency for this unit cell. For that we need to establish a relationship between r and a where r is the radius of the atoms and a is the side of the cube.
Since the atoms present at the face centers are touching the atoms present at the corners of that face, therefore:
a=8r.
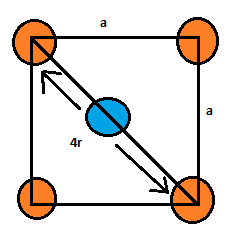
According to the Pythagoras equation:
(4r)2=a2+a2
Therefore, a=8r.
The volume occupied by the atoms present in the unit cell is = 4×34πr3
The volume of the unit cell will be:
Since a=8r, therefore
a3=88r3
The packing efficiency of the unit cell is = 88r34×34πr3=0.74
Hence a face centred cubic unit cell has some voids present in its structure. These voids are octahedral voids and tetrahedral voids. The octahedral voids have a coordination number of six while the tetrahedral voids have a coordination number of four. If there are N atoms per unit cell, then there will be N octahedral voids and 2N tetrahedral voids.
Sodium chloride has a FCC unit cell such that the chloride ions are present at the corners and the face centres of the unit cell and the sodium ions are present at the edge centres of the unit cell and the body centre of the unit cell which are essentially the octahedral voids. The diagram for one face of the unit cell is shown below:
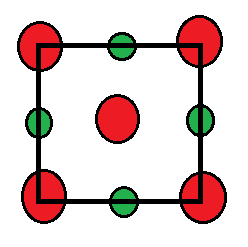
The green coloured ions are sodium ions while the red coloured ions are chloride ions. Now, since sodium ions are present in the octahedral voids, therefore they will be surrounded by six chloride ions.
So, the correct answer is “Option C”.
Note: The coordination number of the chloride ions is also six i.e. each chloride ion is surrounded by six sodium ions. The crystal structure of sodium chloride is CCP (cubic close packed) structure which gives rise to the FCC unit cell.
