Question
Question: How many chiral centres are there in thromboxane \(B2\) ?...
How many chiral centres are there in thromboxane B2 ?
Solution
If a molecule has a non-superimposable mirror image, it is said to be chiral in nature. These compounds have the ability to rotate the plane polarized light to the left or right side, meaning they are optically active compounds.
Complete answer:
Chirality of a carbon means that the carbon would have four different substituent groups attached to it, thus forming a tetrahedral atom. Thus chiral molecules are known to have an asymmetrical centre. Chiral molecules can be identified as non-superimposable mirror images, meaning their mirror images would not match if we were to superimpose or put one above the other.
The chiral centre of a molecule is the atom in which four different functional groups are attached. This means that even if two groups are the same, it won’t qualify as a chiral centre. For instance, the compound named chloro fluoro bromo iodo methane, has a chiral carbon in it.
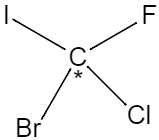
As we can see, the chiral centre in this compound is the carbon at the middle of the compound which has four different groups namely chlorine, bromine, iodine and fluorine attached to it.
Now, we will consider the structure of thromboxane B2.
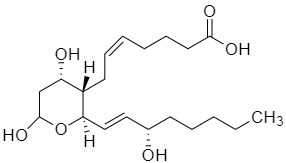
Above given is the structure of thromboxane B2 where the dashes are the bonds which are coming above the plane, and the wedges are going below the plane, in a three dimensional spatial arrangement. As we can see that the molecule has five different chiral centres present in it.
Note: Chiral centres in a molecule are also termed as the steroidogenic centres. The structures, whose mirror images are superimposable with each other are termed as the achiral molecules, in other words they are not chiral.
