Question
Question: How many chiral carbon atoms are there in the open-chain form of glucose?...
How many chiral carbon atoms are there in the open-chain form of glucose?
Explanation
Solution
The chiral carbon atom is the carbon that is attached to four different atoms or groups. The structure of glucose has one aldehyde group at any end of the carbon chain in the open-chain form of glucose. One can try to draw the structure and decide the chiral centers based on atoms attached to the carbon atom.
Complete step by step answer:
- First of all, we will try to understand the meaning of the chiral carbon atom. A chiral carbon atom must be attached to four different atoms or groups. If there are two or more than two same atoms or groups attached then it will not be a chiral carbon.
- Now let us first see the structure of the open chain form of glucose as below,
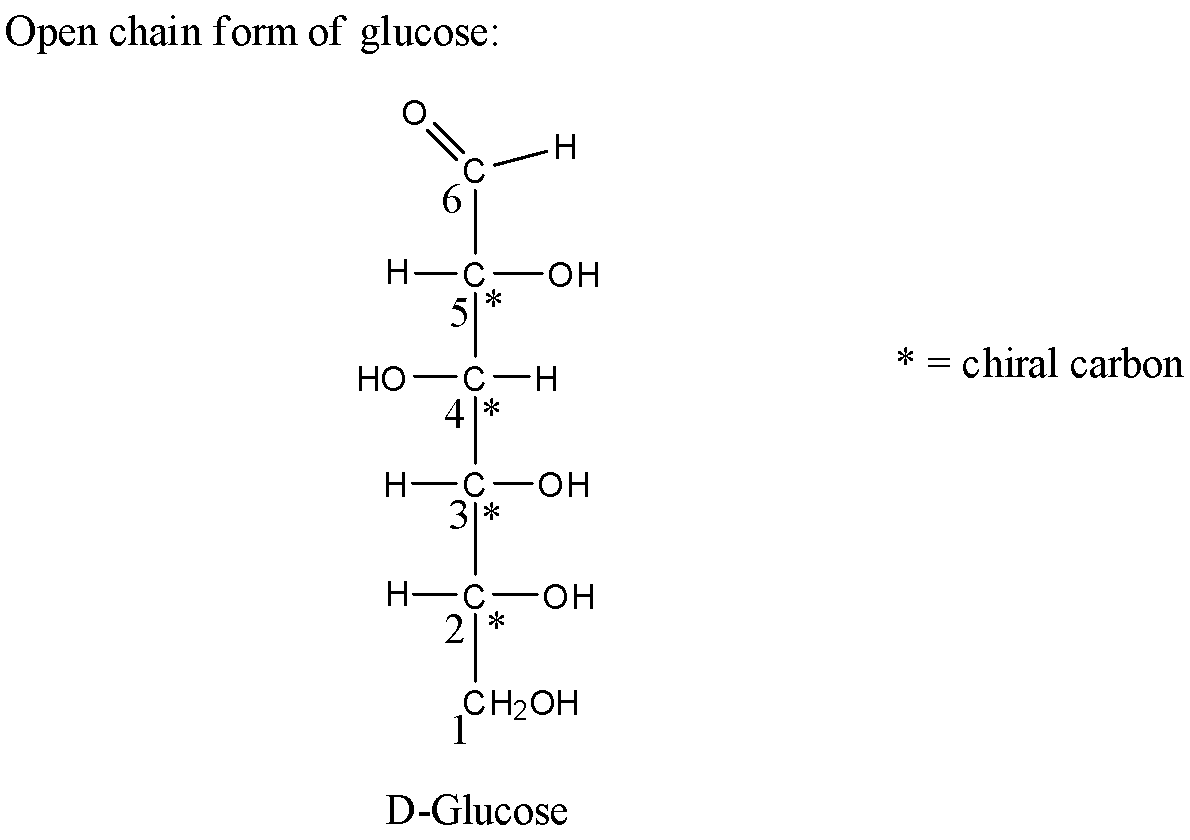
- In the above structure the carbon has been labeled with numbers from below carbon. There is an aldehyde group attached to the 6th carbon atom. Now let us analyze each carbon atom in the above structure and see for the chiral carbon.
- Carbon 1st is attached to two same hydrogen atoms hence it is not a chiral atom. Carbon 2nd is attached to four different groups hence it is a chiral carbon atom. Similarly, carbon 3rd,4th,5th is attached to four different groups hence they are chiral carbon atoms. In the case of carbon, 6th there is a double-bonded oxygen atom attached which means that carbon is not a chiral carbon atom.
Therefore, there in the open-chain form of glucose there are four chiral carbon atoms present.
Note:
In the above structure the symmetry doesn’t pass through the molecule and one should always check this factor while deciding the chiral carbon atom. If the symmetry of the molecule passes through a carbon atom then it is not a chiral carbon atom.
