Question
Question: How many carbocation isomers are possible for the compound with molecular formula \({C_3}H_5^ + \)?...
How many carbocation isomers are possible for the compound with molecular formula C3H5+?
Solution
We must remember that the isomers are compounds having the same chemical formula but different chemical structure. Here we have a compound and we need to find the number of isomers that can be formed. The number of carbons is 3, hence the orientation will be more of chain orientation and not stereo type orientation. Stereo type of orientation is possible in high order carbon compounds.
Complete answer:
Given compound - C3H5+
To determine the number of isomers, we will need to draw the chemical orientation of the given compound.
We can draw the possible orientation for this given compound as,
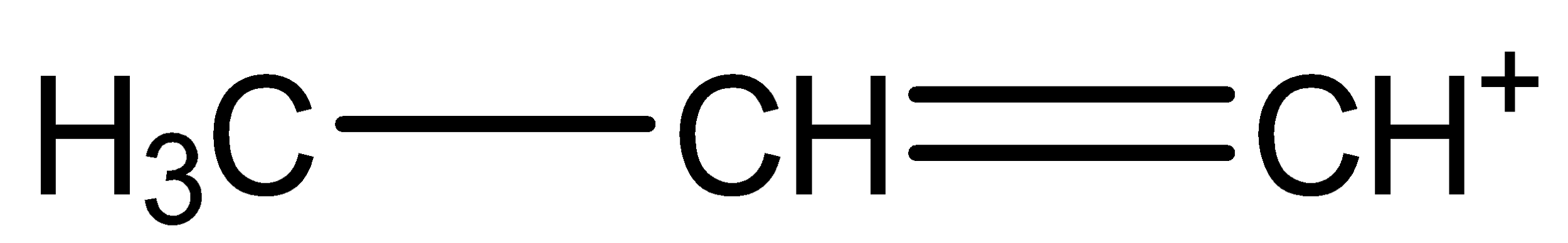
(a)
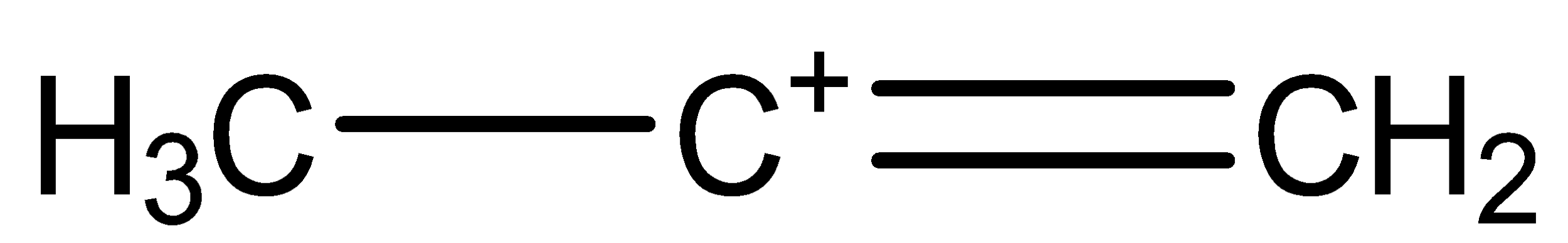
(b)
Here, two orientations are possible as shown in chemical structure (a) and (b).
The (a) chemical structure is called n-propene carbocation.
The (b) chemical structure is called secondary-propene carbocation.
The bonding between the three carbon atoms is one with single bond and one with double bond.
The Hydrogen atoms are arranged by choosing different orientation, keeping one unbonded arm, thus forming a cation.
Hence, the correct answer is 2.
Only 2 isomers are possible for the given carbocation - C3H5+.
Note:
As we know that the isomers are the different structures of the same chemical formula. We must remember that determining the number of isomers that can be formed can be quite tricky at times, especially when the order of carbon atoms is high. But for a lower order carbon compound which is given here. It is quite easy to determine by drawing the different possible orientations.
