Question
Question: How many \(C{O_2}\) will relive in the following reaction? 
Solution
Here, we have to find out the number of carbon dioxide molecules will be eliminated from the given alicyclic compound. The compound given is alicyclic means the compound is aliphatic and it is also a cyclic compound. In the above compound, there is no double bond present in both the rings.
Complete step by step answer:
The given compound is composed of two aliphatic rings, cyclohexane-1, 4-dione and tetrahydrofuran-2, 4 –dione along with two carboxylic acid groups attached to cyclohexane rings.
The structure of both the compounds are shown below.
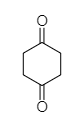
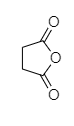
Cyclohexane-1, 4-dione Tetrahydrofuran-2,4-dione
When the derivative of cyclohexane-1, 4-dione is treated with an acid at high temperature, it gives octahydro-2-benzofuran-1,3-dione.
The chemical reaction is shown below.
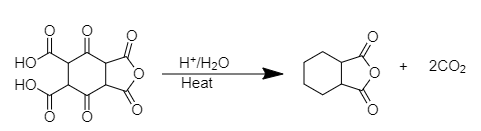
As, we can see from the chemical reaction above, there is an elimination of 2 molecules of carbon dioxide (CO2). The elimination of carbon dioxide molecules lead to the formation of the compound octahydro-2-benzofuran-1,3-dione which contains one cyclohexane ring attached to one molecule of tetrahydrofuran-2, 4-dione.
Therefore, two molecules of CO2 will releave from the compound.
Additional information:
The molecular formula of octahydro-2-benzofuran-1,3-dione is [C8H10O3] and molecular weight is 154.165.The term ‘octahydro’ denotes that there are 8 hydrogen atoms are present in the compound.
The cyclohexane-1,4-dione is an organic compound and is one of the isomers of cyclohexane diones. It is categorised as diketone due to presence of two carbonyl oxygen groups present at 1st and 4th carbon atoms of the ring.
Note: Student should not get confused by the term ’relief’. The term ‘relieve’ denotes the elimination of carbon dioxide from the compound. The elimination of CO2 will also lead to elimination of two carbonyl oxygen atoms from cyclohexane-1,4-dione and 2 carboxylic acid groups attached to the cyclohexane ring.
