Question
Question: How many bridging oxygen atoms are present in \({P_4}{O_{10}}\)? (A) 6 (B) 4 (C) 2 (D) 5...
How many bridging oxygen atoms are present in P4O10?
(A) 6
(B) 4
(C) 2
(D) 5
Solution
Phosphorus pentoxide, P4O10, is a dimer of the compound phosphorus (V) oxide, P2O5. It is a covalent compound. Phosphorus is basically a pentavalent element and it reacts with oxygen to form oxides such as P2O3 and P4O10. Both of these oxides of phosphorus exist as dimer that means these compounds are formed by a combination of two molecules of that compound. The compound P4O10 is a very good dehydrating agent.
Complete step by step solution: To find out the number of bridging oxygen atoms in P4O10, we need to first understand what does a bridging atom means. Further, we need to draw and understand the chemical structure of P4O10.
In P4O10, there are 4 phosphorus atoms and 10 oxygen atoms. Each phosphorus atom in P4O10 is linked to 4 oxygen atoms.
The diagram given below is the structure of P4O10.
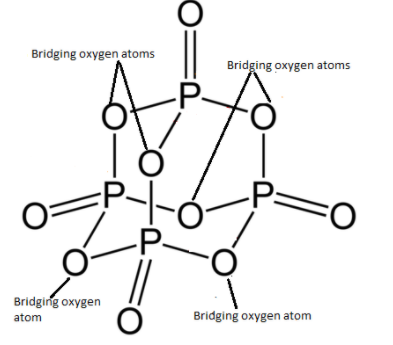
As you can see, the phosphorus atoms and oxygen atoms bonded to form a closed cage like structure. There are six P−O−P bonds and each oxygen atom undergoes sp3 hybridisation. There are 4 P=O where hybridisation of oxygen is sp2. As we are aware of the fact that each oxygen will have two lone pairs of electrons. Therefore, the total number of lone pairs of electrons in P4O10 are 20.
As there are six P−O−P bonds present in P4O10, these six oxygen atoms combine two tetrahedrons together. These 6 oxygen atoms are known as bridging oxygen atoms.
Therefore, six bridging oxygen atoms are present in P4O10.
Hence, the correct answer is option (A).
Note: In P4O10, oxygen is bridging atom which help to combine two molecules of P2O5 to form a dimer. Here, we can say that the monomer is P2O5. The oxygen atom is a bridging atom. Students should not confuse the term ‘bridging atom’ with ‘ligand’. Ligand is the term used in coordination chemistry. A ligand can be an ion or molecule and it binds the central metal atom or ion to form coordination complexes. It can be anion or cation or sometimes sit can be a neutral molecule. The examples of ligands include NO+, NH3, H2O. The bridging ligand will bound more than one metal atom in the coordination complex.
