Question
Question: How many bonds do carbon forms in neutral compounds?...
How many bonds do carbon forms in neutral compounds?
Solution
First of all we should know about carbon, its properties and bond formation with other compounds. Carbon is a chemical element whose atomic number is six with the symbol as C. It is a non-metal that generally shows tetravalency, which means that the maximum bond formed by carbon is only four.
Complete step-by-step answer: About carbon atom:-
General characteristic of carbon atom:-
-It is abundant, common.
-It generally forms strong covalent bonds.
-It has four valence electrons, hence it will form four bonds with other compounds.
-It has a variety of shapes.
-It generally forms bonds with multiple elements.
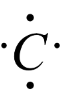
Chemical properties of carbon atom are as follows:-
-Its chemical formula is C.
-Oxidation: It generally combines with the oxygen to produce carbon dioxide and carbon monoxide.
-Reactivity: It does not react or dissolve in water or acids.
-Chains of Atoms: It has the ability to make long chains of atoms.
Uses of carbon atom:-
-The human body comprises 18% of the total carbon atom.
-It is also found in sugar, glucose, proteins etc.
-Its isotope is diamond which is generally used in making jewelry.
-Amorphous carbon is used to make inks and paints.
-Graphite is an isotope of carbon which is generally used as the lead in pencils.
-Carbon dating is one of the most important uses of carbon atoms.
-Carbon is widely used in industries.
Carbon generally forms four covalent bonds with neutral atoms, because of having four valence electrons in its outermost shell or orbit.
Note: Carbon has an exceptional ability to form bonds with a large variety of bonds. It generally shows anomalous behavior due to various properties such as high ionization energy, high electrification, exceptional size and absence of d-orbitals.
