Question
Question: How many bonds are present in \[S - S\] bonds in pyrosulphuric acid (oleum)?...
How many bonds are present in S−S bonds in pyrosulphuric acid (oleum)?
Solution
Pyrosulphuric acid or disulfuric acid is the main constituent of fuming sulfuric acid. It is a solution of sulfur trioxide (SO3) in anhydrous sulfuric acid. Pyrosulphuric acid is a colourless liquid that fumes in moist air due to the reaction between SO3 and H2O, which leads to the formation of poorly volatile H2SO4. It is also used for sulfation of organic compounds.
Complete answer:
We can determine the number of S−S bonds present in pyrosulfuric acid (oleum) by taking a look at its structure.
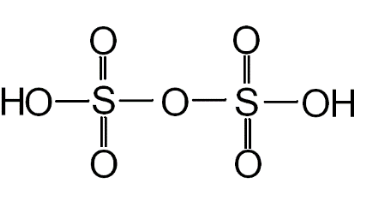
Fig: Structure of pyrosulfuric acid (oleum).
From the above structure we can easily figure out that there are no S−S bonds present in oleum.
Therefore, the number of bonds present in S−S bonds in pyrosulfuric acid (oleum) is zero.
Note:
Oleum is a harsh reagent and is highly corrosive. Due to the high enthalpy of oleum it is used as an important intermediate in the manufacture of sulfuric acid. It is also used as a reagent in the secondary nitration of nitrobenzene.
