Question
Question: How many atoms of \(XeO_{6}^{-4}\) lie in the same plane?...
How many atoms of XeO6−4 lie in the same plane?
Solution
XeO6−4 is known as perxenates which has octahedral molecular geometry and it was determined by Raman spectroscopy. We can synthesize perxenate by the disproportionation of xenon trioxide when it is dissolved in strong alkali. They are also strong oxidising agents.
Complete answer:
Synthesis of perxenates is done by disproportionation of Xenon trioxide when it is dissolved in strong alkali. In this, Ba(OH)2 is an alkali.
To calculate the hybridization of XeO6−4 , firstly we should know the valence electron for Xe which is equal to 8 .
The covalency of Xe is 8
The number of sigma bond is 6 and lone pair is 0
To calculate the number of hybrid orbitals,
H.O=σbond+l.p
where, H.O is the hybrid orbital, σbond is the sigma bond and lp is the lone pair on the central atom.
Now, substituting the values in the given formula, we get,
H.O=6+0
⇒H.O=6
Therefore, the hybridization for XeO6−4 is sp3d2
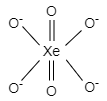
And the geometry formed is octahedral.
In XeO6−4 structure, two oxygen atoms form double bonds and four oxygen atoms form single bonds to complete the octet of xenon.
XeO6−4 is a strong oxidizing agent. In acidic solution, perxenate ion is unstable.
XeO6−4 has a hybridization of sp3d2 and the shape of its molecule is octahedral. It has five atoms in one plane, that is, four oxygen and one xenon.
Hence, the number of atoms of XeO6−4 in the same plane is 5 .
Note: Xenon having +8 charge does not have an outer valence electron for lone pairs, therefore, the number of lone pairs on the central atom is zero.
-In the structure of XeO6−4 , oxides are equivalent.
-The number of sigma bonds in XeO6−4 is six.
