Question
Question: How many atom(s) of \({\text{S(C}}{{\text{H}}_{\text{2}}}{\text{)}}{{\text{F}}_{\text{4}}}\) may lie...
How many atom(s) of S(CH2)F4 may lie in the equatorial plane?
Solution
The vertical bonds are known as the axial bonds whereas the horizontal bonds are known as the equatorial bonds. The axial bonds are parallel to the plane of the molecule while the equatorial bonds are perpendicular to the plane.
Complete step by step answer:
-The structure of S(CH2)F4 is as follows:
The central atom is the sulfur atom. The four fluorine groups are attached to the central sulfur atom by single bond. And the methylene group is attached to the central sulfur atom by a double bond. Thus, the structure of S(CH2)F4 is,
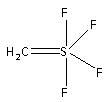
-In the structure of S(CH2)F4 the one methylene group and two fluorine atoms are attached in such a manner that they form a trigonal structure. The remaining two fluorine atoms are attached like the two pyramids. Thus, the structure of S(CH2)F4 is trigonal bipyramidal.
-In trigonal bipyramidal structure of S(CH2)F4, the two fluorine atoms are placed vertically and are parallel to the plane of the molecule. Of the other two fluorine atoms, one fluorine atom lies to the right side of the plane while the other lies to the left side of the plane. The methylene group is attached horizontally and is perpendicular to the plane of the molecule.
Thus, S(CH2)F4 has one atom in the equatorial plane.
Note: The hydrogen atoms are attached to the carbon atom of the methylene group and not to the central sulfur atoms. The hydrogen atoms lie on the right and left sides of the plane. Thus, the hydrogen atoms are neither axial or equatorial.
