Question
Question: How many allotropes of dihydrogen are known? What is their importance?...
How many allotropes of dihydrogen are known? What is their importance?
Solution
Dihydrogen is formed from two hydrogen atoms. It is a homonuclear diatomic molecule. It contains the covalent bonds between the two hydrogen atoms. Dihydrogen is the lightest of all the molecules known. The allotropes of dihydrogen differ in nuclear spins.
Complete answer: The molecular formula of dihydrogen is H2. Dihydrogen molecule consists of two hydrogens attached with covalent bonds. There are two allotropes of dihydrogen known. These allotropes are:
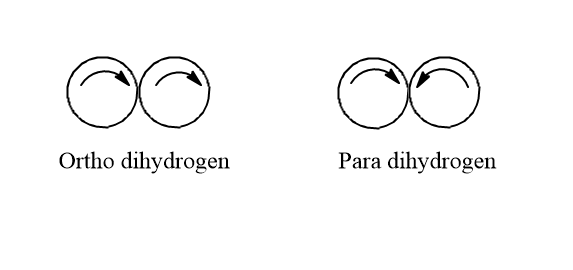
Ortho-dihydrogen and para-dihydrogen.
The difference between ortho-dihydrogen and para-dihydrogen is in the nuclear spin of both atoms.
Ortho-dihydrogen- in this hydrogen molecule the protons present in the nuclei of both the hydrogen atoms are present in the same spin. i.e. They spin in the same direction. The resultant nuclear spin is one. It has both inter and intramolecular hydrogen bonding.
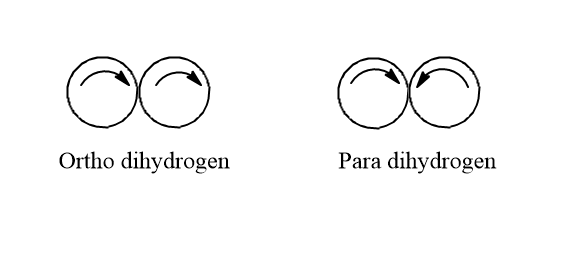
Para-dihydrogen- in this dihydrogen the protons present in the nuclei of both the hydrogen atoms are present in the opposite spin i.e. they spin in opposite direction. In this, the resultant nuclear spin is zero as the spins cancel out each other. It has only intermolecular hydrogen bonding.
The importance of allotropes of dihydrogen is in economic areas. The dihydrogen contains 25% of parahydrogen and 75% of ortho hydrogen. The space vehicles use liquid hydrogen as a fuel; in them it mostly has para dihydrogen. In today’s time, modern liquefaction techniques are being developed to convert ortho forms to para forms so that they can be used economically.
Note:
Ortho-dihydrogen is much stable at room temperature and para-dihydrogen is much stable at very low temperature. It exists as a colorless, tasteless, and odorless gas which is highly combustible. The percentage of both the allotropes 25% and 75% is at room temperature.
