Question
Question: How many alkenes on catalytic hydrogenation give isopentane as a product? (a) 2 (b) 4 (c) 3 ...
How many alkenes on catalytic hydrogenation give isopentane as a product?
(a) 2
(b) 4
(c) 3
(d) 5
Solution
Isopentane is an organic chemical compound in which there is a chain of four carbon atoms and the second carbon atom has a methyl group attached. Catalytic hydrogenation of alkene means the double bond is converted into a single bond by the addition of a hydrogen atom in the presence of metals like palladium or platinum.
Complete step by step answer:
Isopentane is an organic chemical compound in which there is a chain of four carbon atoms and the second carbon atom has a methyl group attached. Since it has the only single bond between all the carbon atoms it is an alkane. The formula is given below:
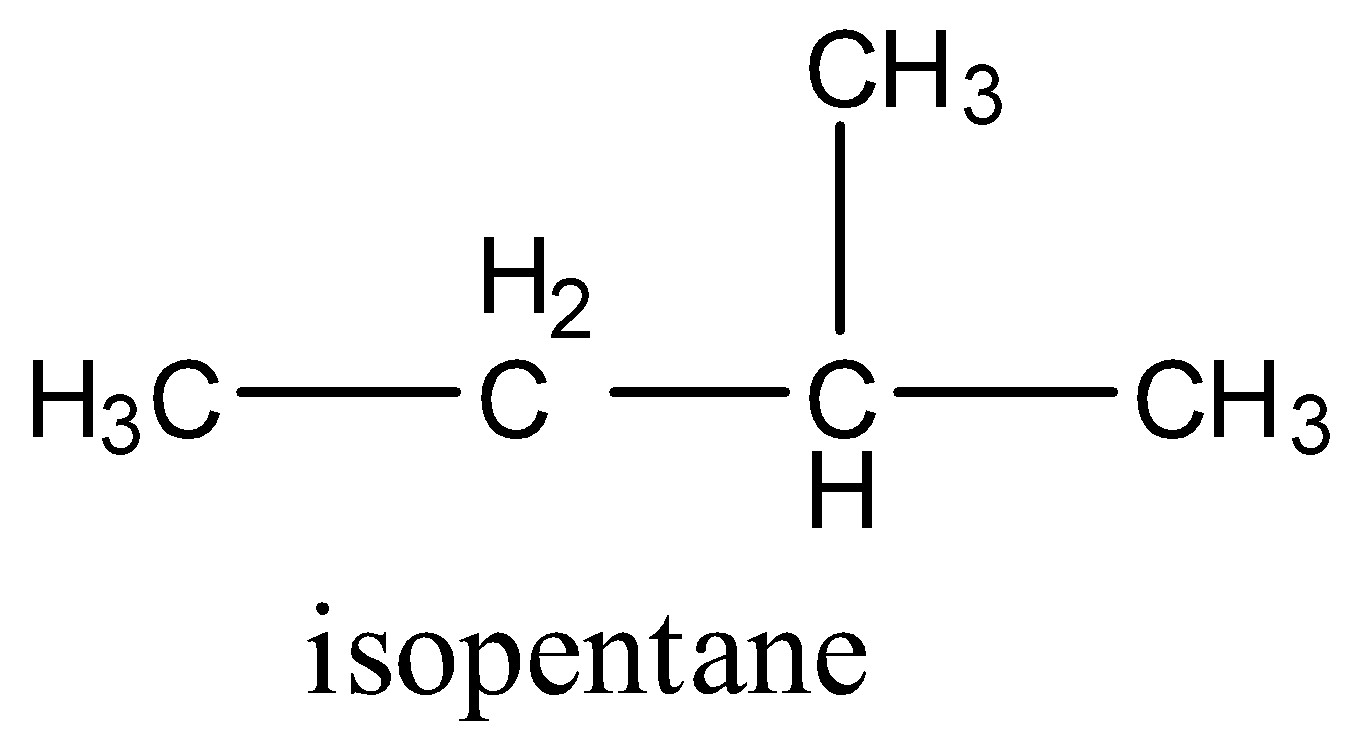
Catalytic hydrogenation of alkene means the double bond is converted into a single bond by the addition of a hydrogen atom in the presence of metals like palladium or platinum. So three molecules can give isopentane on catalytic hydrogenation.
(a)- 3-Methyl butene
It has a structure:
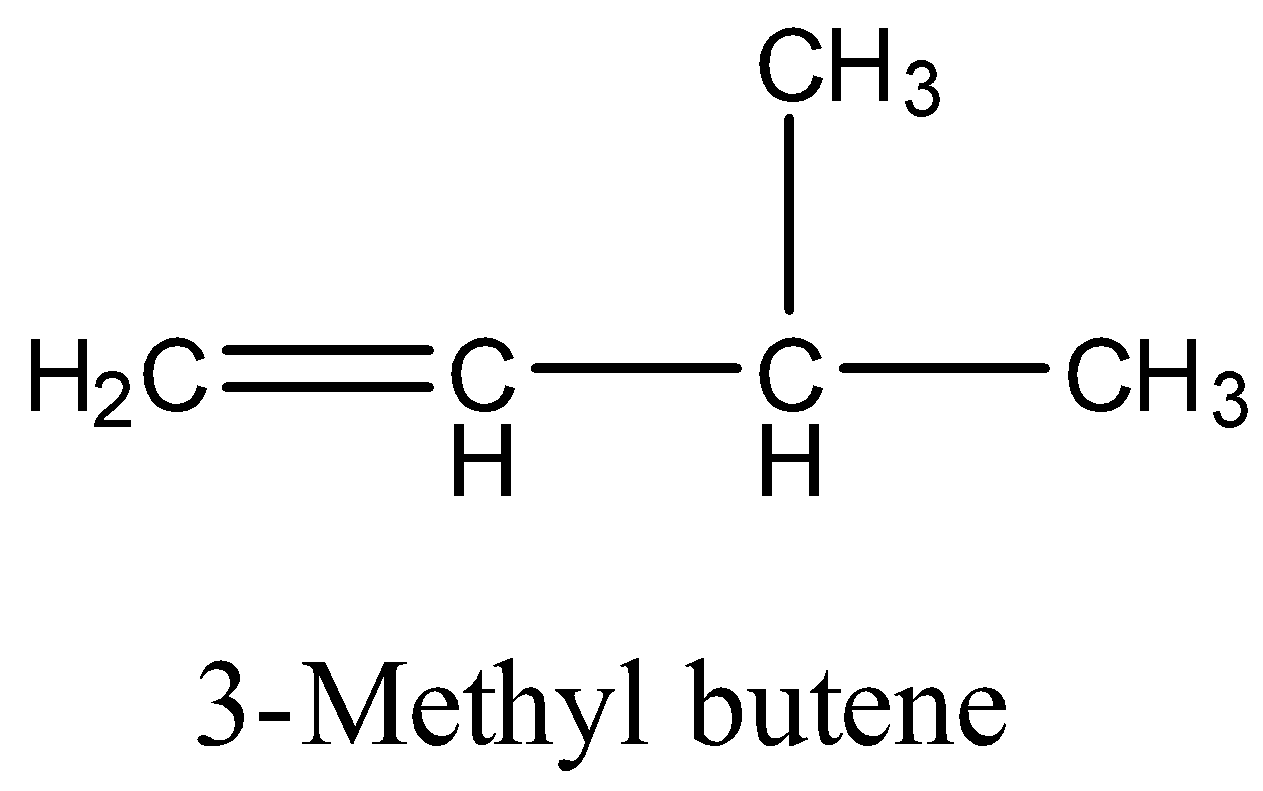
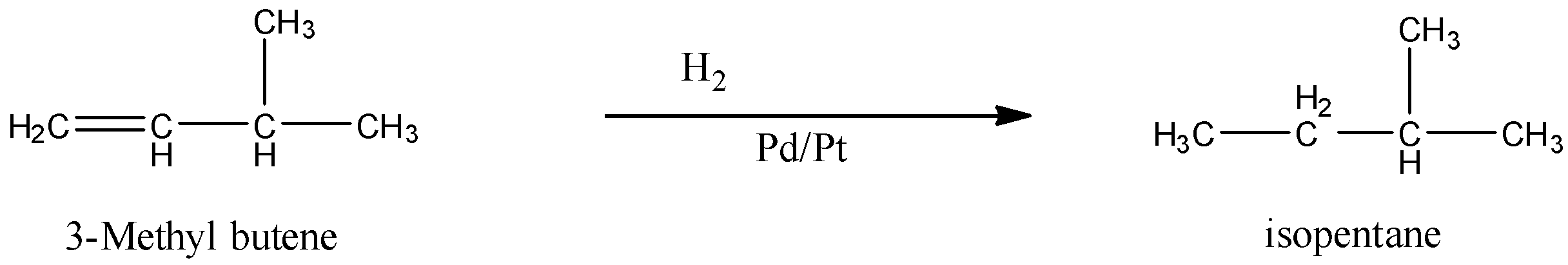
When 3-Methyl butene is subjected to catalytic hydrogenation then the product is isopentane. The reaction is given below:
(b)- 2-Methyl but-2-ene
It has a structure:
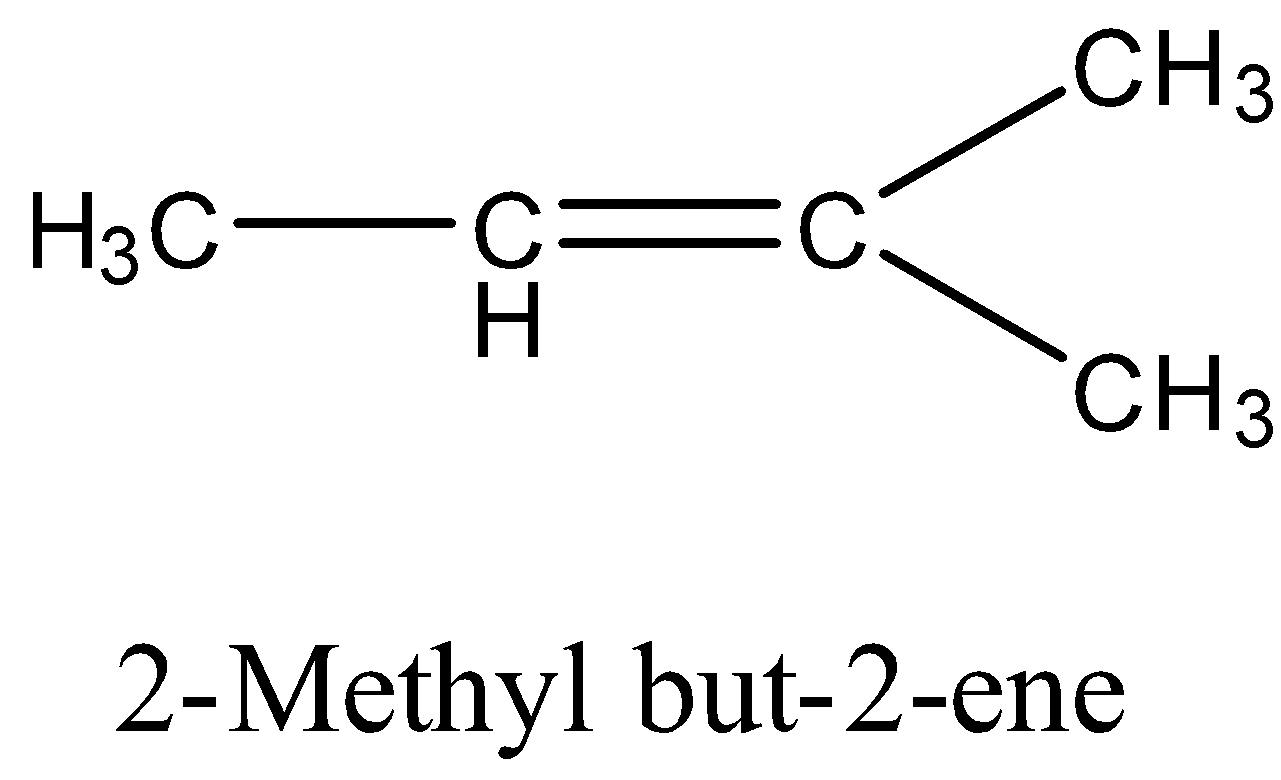
When 2-Methyl but-2-ene is subjected to catalytic hydrogenation then the product is isopentane. The reaction is given below:
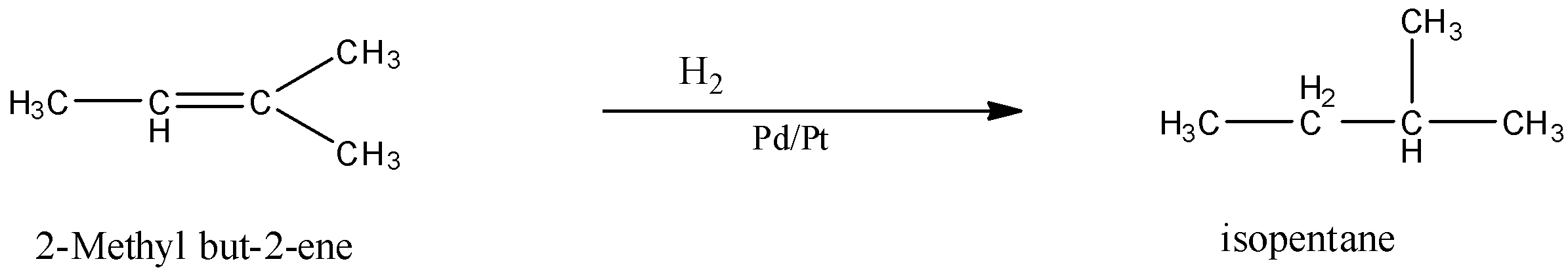
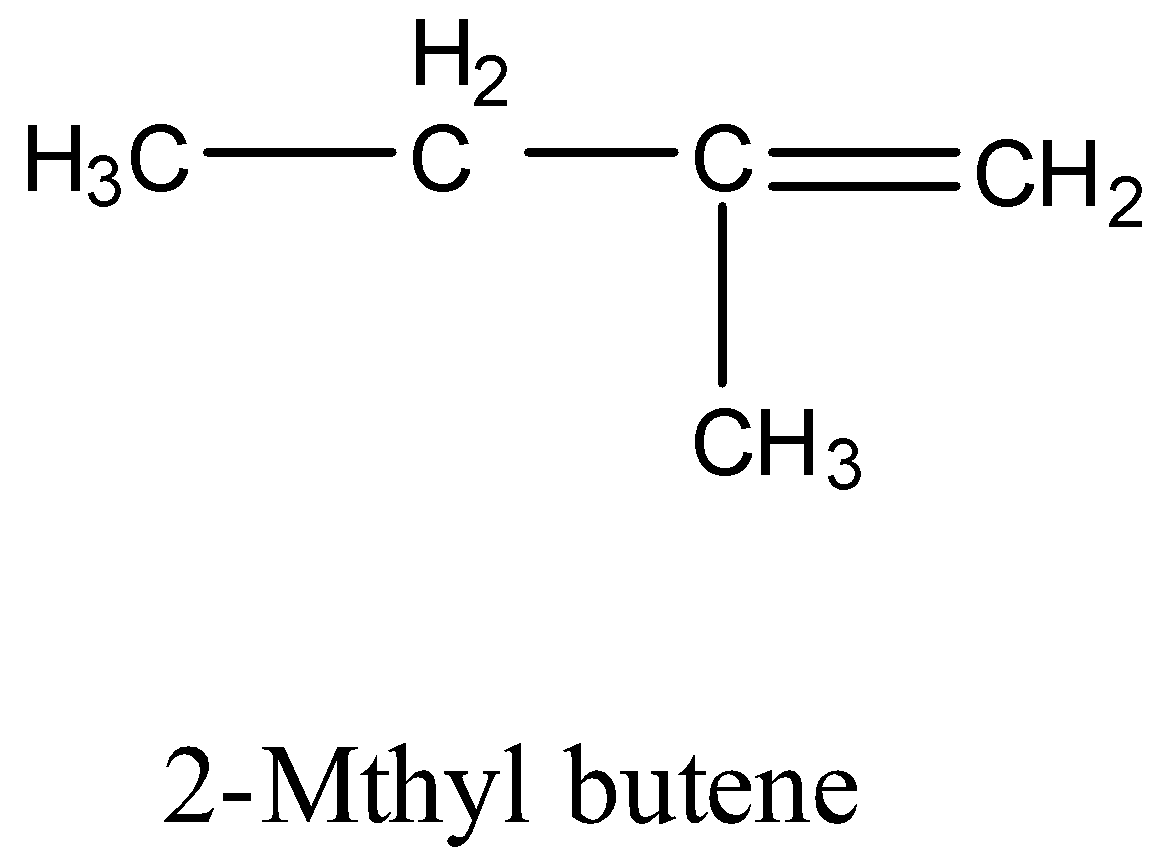
(c)- 2-Methyl butene
It has a structure:
When 2-Methyl butene is subjected to catalytic hydrogenation then the product is isopentane. The reaction is given below:
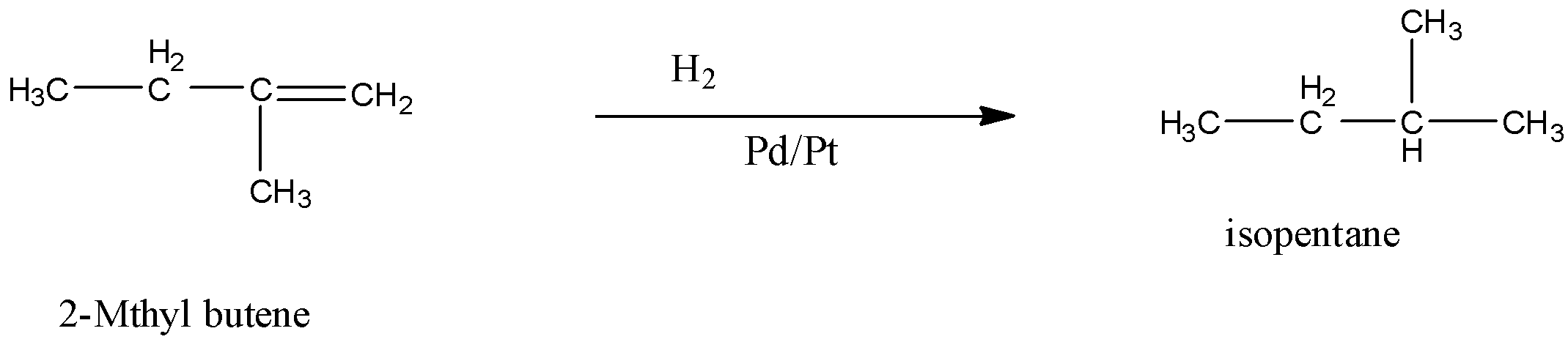
So, the correct answer is “Option C”.
Note: This is the easiest method to convert an alkene or an alkyne into an alkane. These reactions are also known as Sabatier and Senderens reaction. But the catalyst used in this reaction (palladium or platinum is very costly).
