Question
Question: How many acids can we use to make iso-butane by decarboxylation? A. \( 4 \) B. \( 3 \) C. \(...
How many acids can we use to make iso-butane by decarboxylation?
A. 4
B. 3
C. 2
D. 5
Solution
An organic reaction in which removal of substituent groups takes place, is known as elimination reaction. Decarboxylation reaction is the type of elimination reaction in which a carboxylic acid is reduced to respective alkane with the removal of carbon dioxide.
Complete answer:
The structure of given compound i.e., isobutane is as follows:
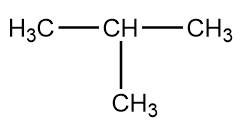
The formation of isobutane by the decarboxylation reactions can take place in following ways:
Preparation Reaction-1:
When 2-methylbutanoic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
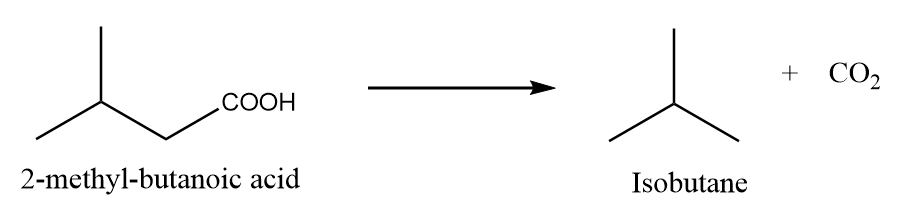
Preparation Reaction-2:
When 2,2-dimethylpropanoic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
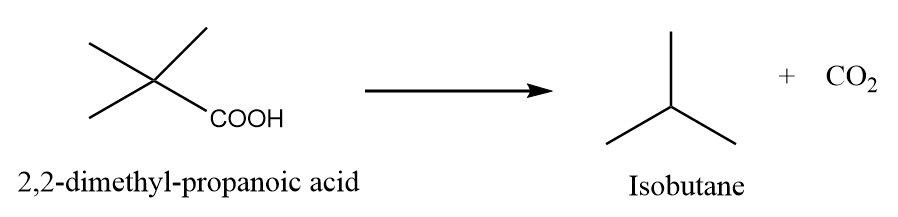
Preparation Reaction-3:
When 3-methylpentanedioic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
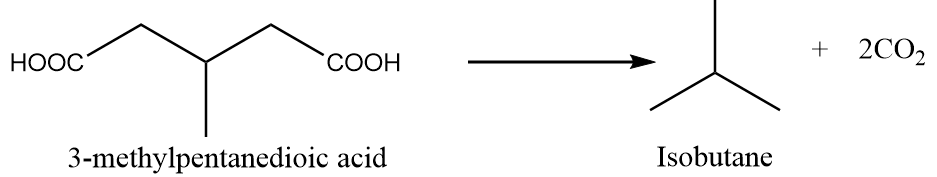
Preparation Reaction-4:
When 2,2-dimethylbutanedioc acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
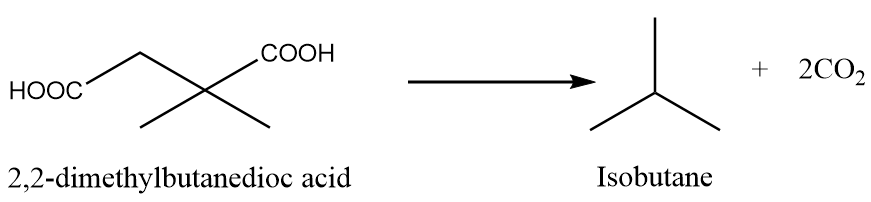
Preparation Reaction-5:
When 2-methylpropane-1,2,3-tricarboxylic acid is heated under standard conditions, then the formation of isobutane takes place along with the removal of carbon dioxide gas. The reaction is as follows:
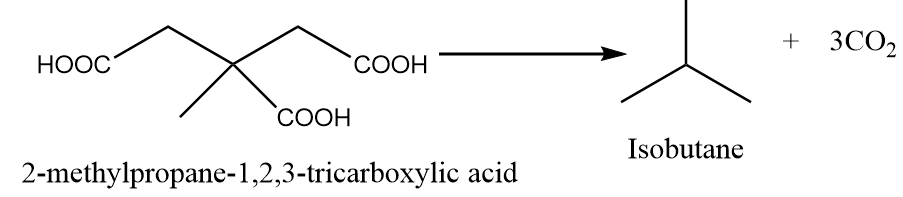
Hence, five acids can be used to form isobutane via decarboxylation reaction.
Thus, option (D) is the correct answer.
Note:
It is important to note that in actuality, there are a total seven acids through which isobutane can be formed via decarboxylation reactions which are two monobasic acids, three dibasic acids and two tribasic acids. It is considered as the most important reaction for the formation of alkanes.
