Question
Question: How many 3C - 4\({{\text{e}}^{-}}\) bonds are present in the dimer of \(\text{BeC}{{\text{l}}_{2}}\)...
How many 3C - 4e− bonds are present in the dimer of BeCl2?
Solution
3C-4e− bonds stands for three centre - 4 electron bonds. Coordination bonds are those bonds which are made after the sharing of lone pair electrons. 3C-4e− bond means that in between 3 atoms total 4 electrons are shared which do not participate in the reaction.
Complete answer:
-Firstly, we have to draw the structure of beryllium chloride.
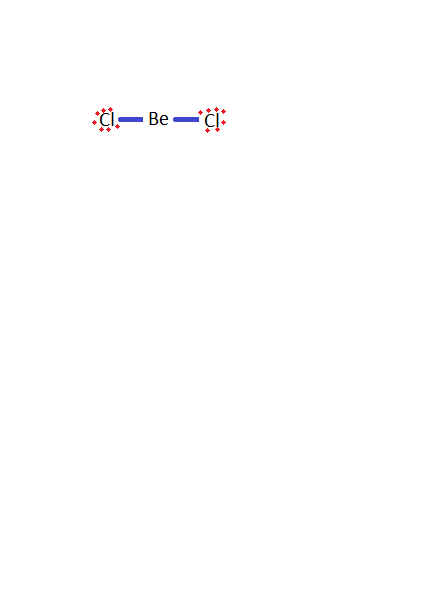
-Now, here chlorine has a lone pair of an electron which can be shared with the other molecules which are electron deficient.
-So, beryllium chloride dimerises to form Be2Cl4 because it is unstable due to incomplete octet present in the boron and the structure of it is:
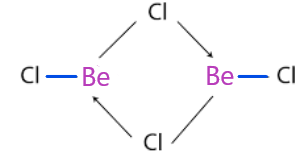
-So, we can see that the two molecules of chlorine share the lone pair of an electron with boron to fulfill the octet.
-Also, the compound is an electron-deficient molecule because electron-deficient molecules are those in which the total no. of atoms in the boron is less than 8.
-As we can also see that four-electrons are being shared, that is two electrons from each chlorine atom and they are given to the boron.
-So, a total of 3 atoms participated that's why it is called 3 centre - 4 electron bond.
-And a total of two bonds are formed.
Therefore, total 2, 3C - 4e− bonds in the structure of beryllium chloride.
Note: Electron deficient molecules tend to accept electrons so they can make coordinate bonds easily. Other molecules which can form 3C - 4e−bonds are XeF2, Al2Cl6, HF, etc. Electron rich molecules are those species which have an extra pair of electrons to make the bond with electron deficient molecules.
