Question
Question: How many 3 member ring isomers are possible in \[{C_4}{H_6}C{l_2}\] ? A. 4 B. 2 C. 6 D. 5...
How many 3 member ring isomers are possible in C4H6Cl2 ?
A. 4
B. 2
C. 6
D. 5
Solution
Isomers are compounds with the same molecular formula but the different structural formulas and different properties/arrangements in space. Isomerism is the process of holding isomers and it can be classified into different types as structural, functional, stereo, and geometrical isomerism based on its structure, arrangement, and properties.
Complete step by step answer:
Three-member rings are called cyclopropane. The possible isomers present in the compound with molecular formula C4H6Cl2 can be drawn below.
Generally, a carbon atom has valency four, and thus, it forms four bonds with other atoms. The valency of chlorine is one and thus, it forms one bond with other atoms.
The first possible isomer can be drawn as,
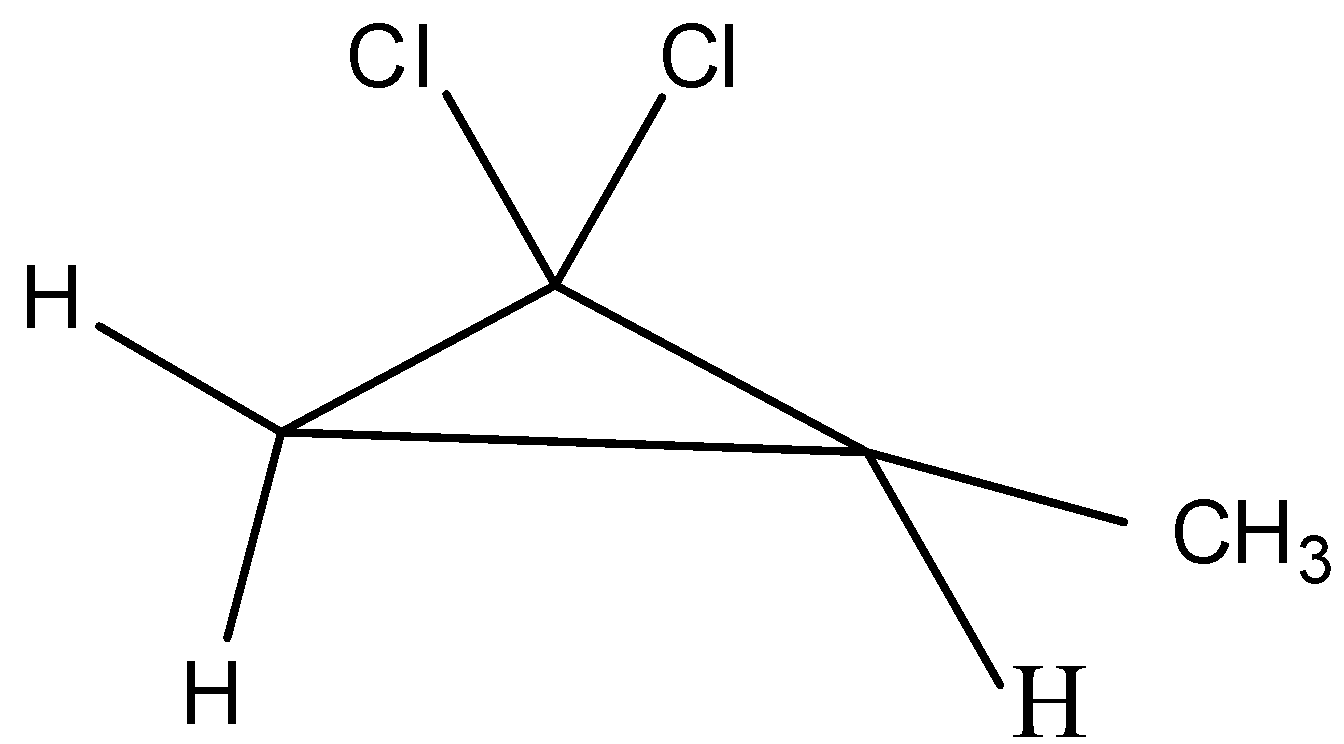
The name of the above structure is 1,1-dichloro-2-methylcyclopropane and it has the molecular formula C4H6Cl2.
The second possible isomer can be drawn as,
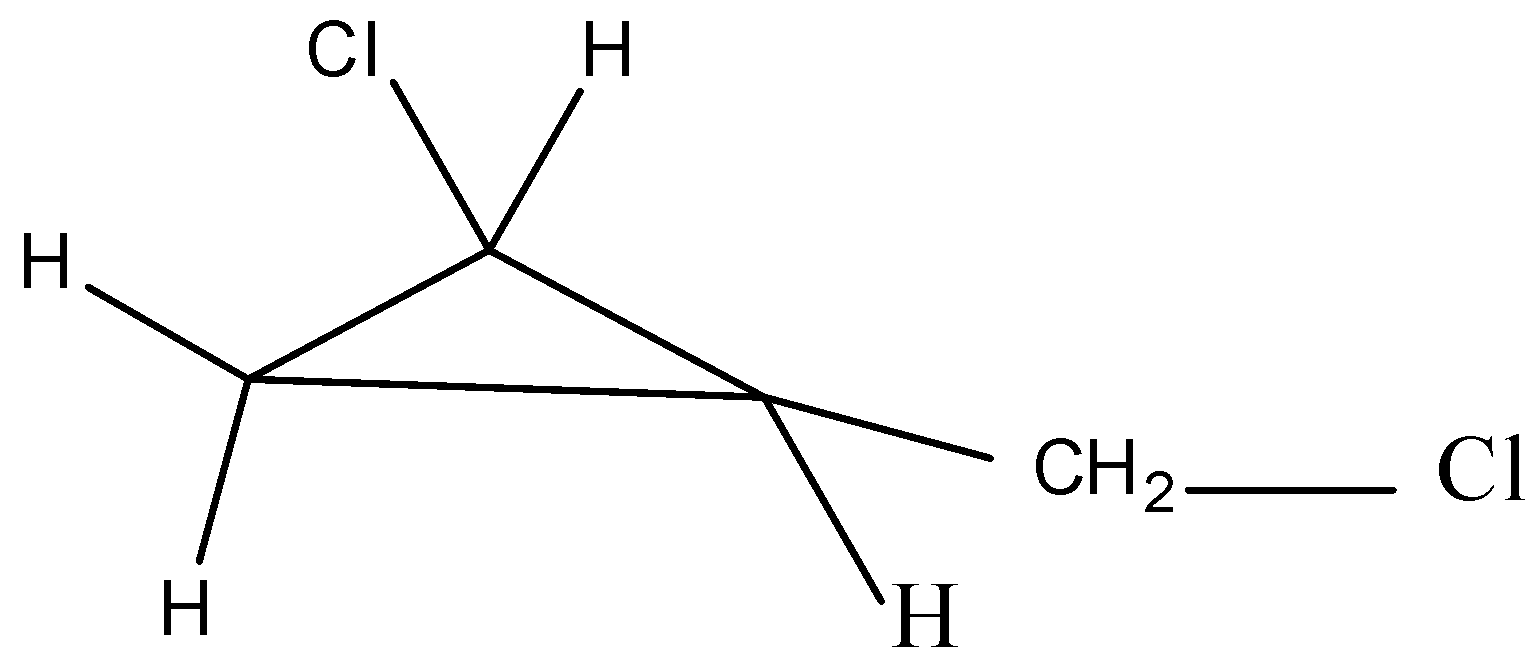
The name of the above structure is 1-chloro-2-(chloromethyl)cyclopropane and it has the molecular formula C4H6Cl2.
The third possible isomer can be drawn as,
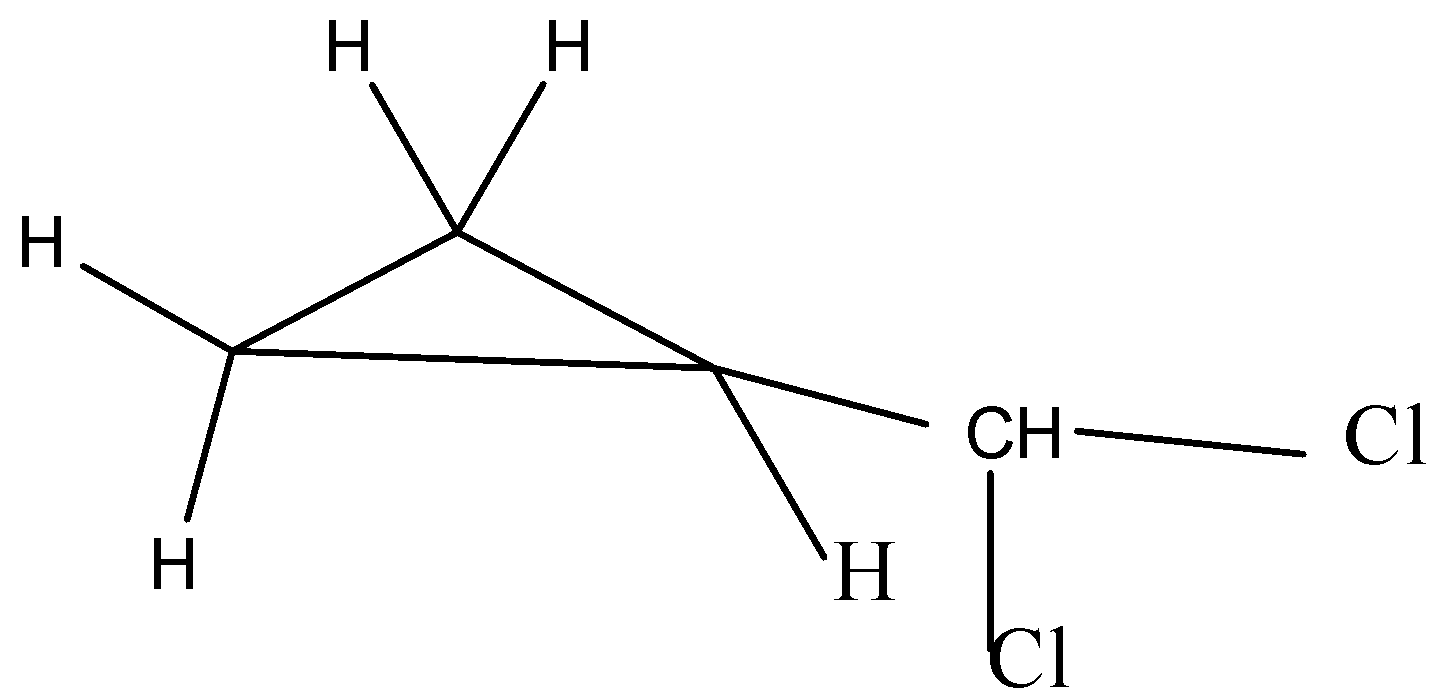
The name of the above structure is 1-(dichloromethyl)cyclopropane and it has the molecular formula C4H6Cl2.
The fourth possible isomer can be drawn as,
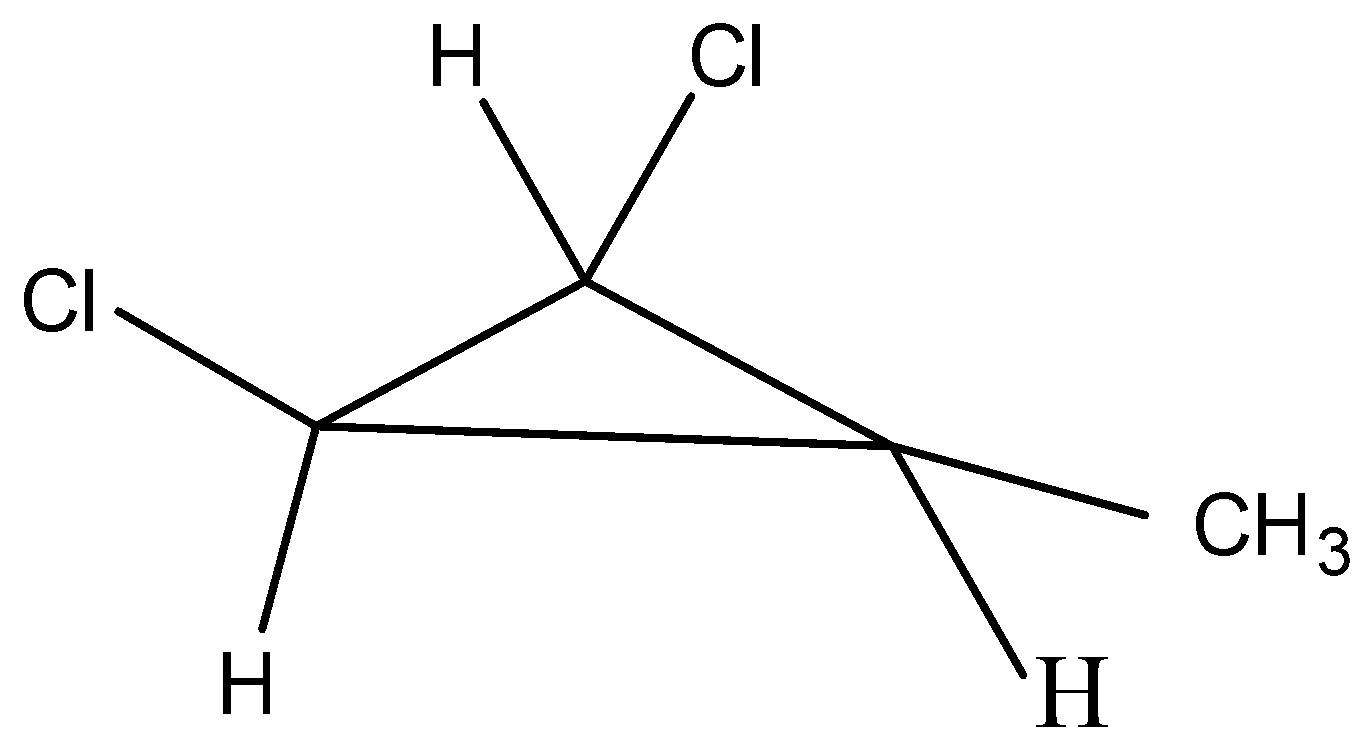
The name of the above structure is 1-chloro-2-chloro-3-methyl cyclopropane and it has the molecular formula C4H6Cl2.
The fifth possible isomer can be drawn as,
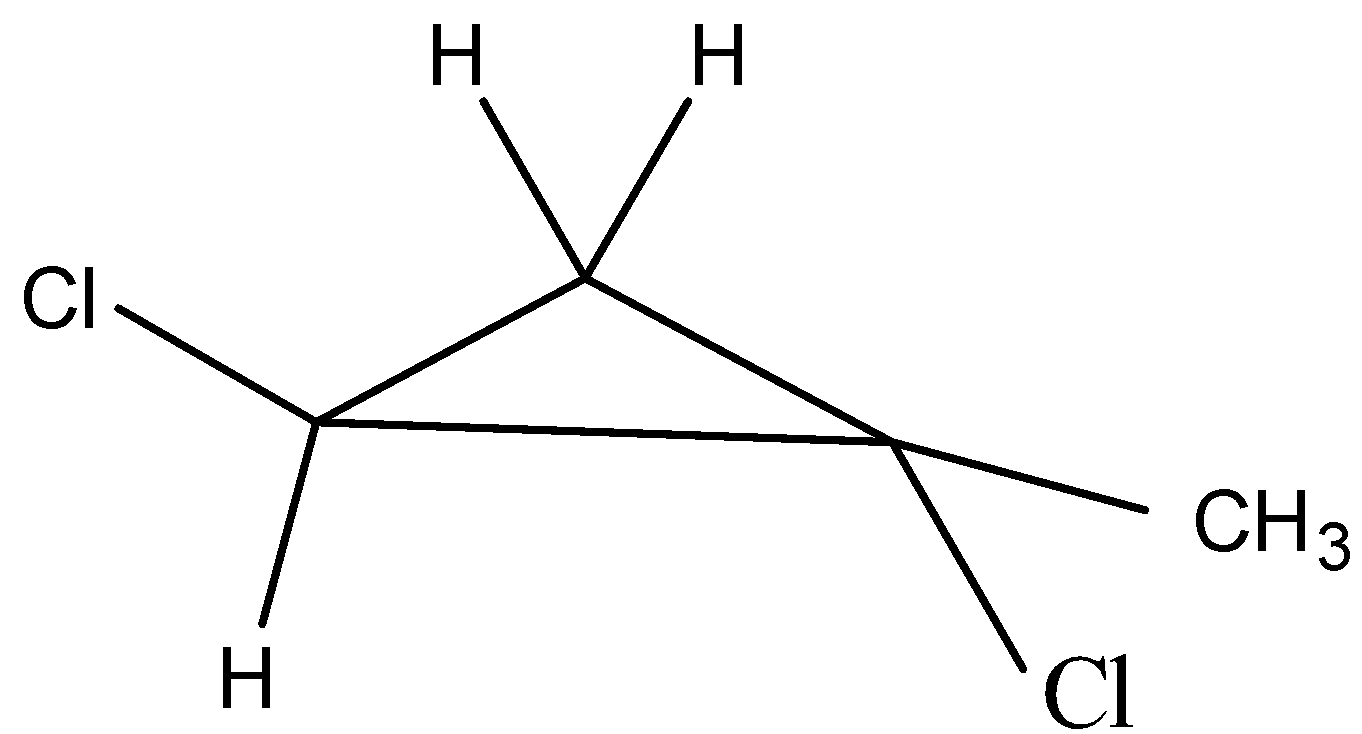
The name of the above structure is 1-chloro-1-methyl 2-chloro- cyclopropane and it has the molecular formula C4H6Cl2.
The sixth possible isomer can be drawn as,
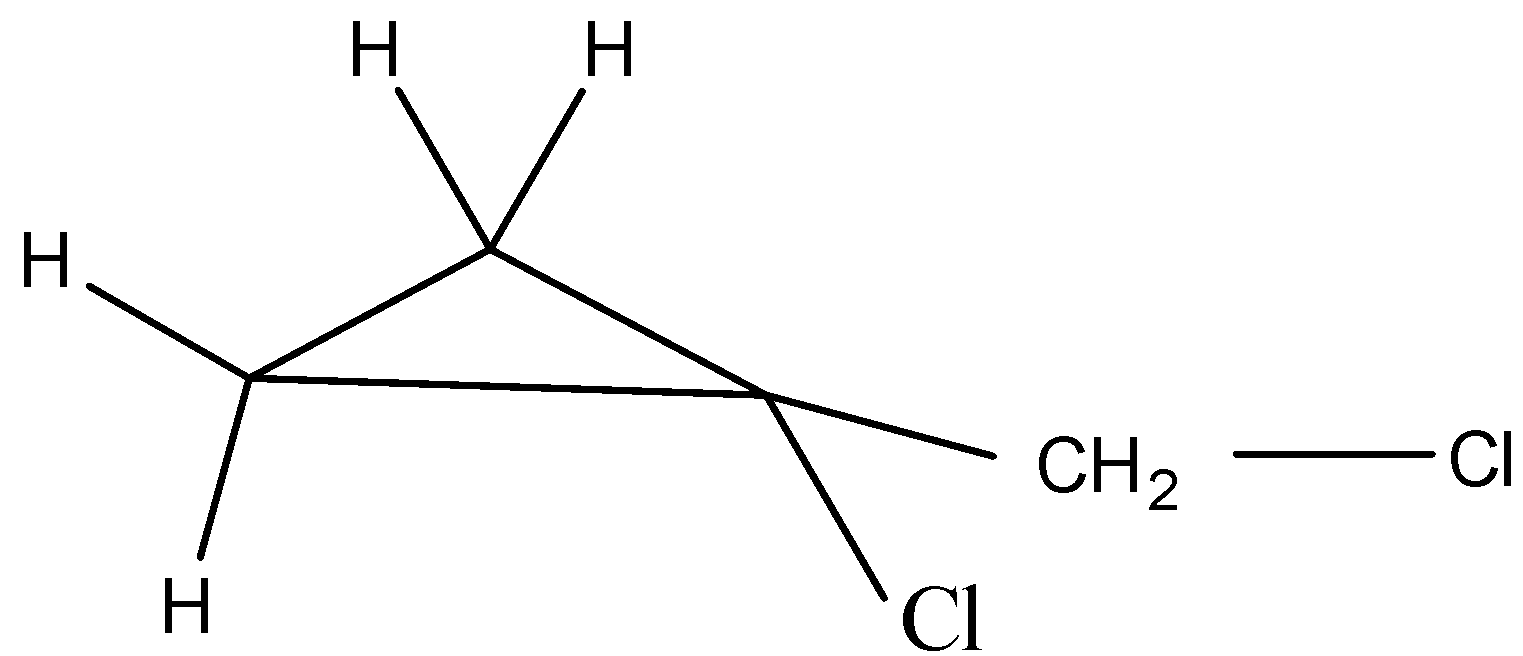
The name of the above structure is 1-chloro-1-(chloromethyl) cyclopropane and it has the molecular formula C4H6Cl2.
From the above structures, it is clear that six isomers are possible in 3 member ring of molecular formula C4H6Cl2
Therefore, the correct option is C.
Note: Structural isomers are the compound with the same molecular formula but different structural formula. Under structural isomerism, functional, chain, and tautomers are present. Stereoisomers are the compound with the same molecular formula but different orientation in space. Under stereoisomers, geometry and optical isomers are present.
