Question
Question: How is sodium nitrate prepared from sodium hydroxide?...
How is sodium nitrate prepared from sodium hydroxide?
Solution
Hint : A chemical reaction in which an acid reacts with a base to form a salt and hydrogen ions and hydroxide ions released by acid and bases to generate water is known as a neutralization reaction. The salt formed by the neutralization of a strong acid and strong base has a pH equal to 7 , salt formed by the neutralization of a strong acid and weak base has a pH less to 7 and salt formed by the neutralization of a weak acid and strong base has a pH greater than 7 .
Complete Step By Step Answer:
Sodium nitrate is an inorganic salt of alkali metal whose chemical formula is NaNO3 . It is commonly referred to as “Chile saltpetre” and on dissociation it gives sodium cation i.e., Na+ and a nitrate ion i.e., NO3− . At room temperature, it exists as a white solid which is crystalline and highly soluble in water. It is a non-flammable compound but reacts violently when reacted with flammable compounds. The structure of sodium nitrate is as follows:
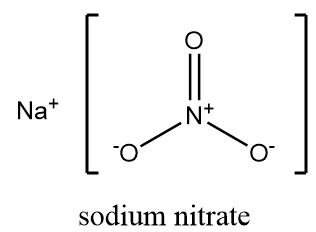
Sodium nitrate can be prepared from sodium hydroxide by a neutralization reaction. As sodium hydroxide is a strong base, it reacts with nitric acid and formation of sodium nitrate takes place along with the removal of water molecules. The reaction takes place as follows:
NaOH+HNO3→NaNO3+H2O .
Note :
It is important to note that the reaction between nitric acid and sodium hydroxide is a highly exothermic acid as the reaction between strong acid and strong base takes place. Alternatively, sodium nitrate can be formed from sodium hydroxide by reacting it with ammonium nitrate. The reaction is as follows:
NaOH+NH4NO3→NH4OH+NaNO3
