Question
Question: How is propanone converted into (i) \[propan - 2 - ol\] (ii) \[2 - methylpropan - 2 - ol\]...
How is propanone converted into (i) propan−2−ol (ii) 2−methylpropan−2−ol
Solution
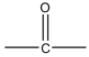
Propanone is an organic compound with a chemical formula C3H6O. It is the simplest and smallest member of the ketone family. Its common name is acetone. Acetone is a colorless, highly volatile, and flammable liquid. It has a very pungent odour.
Complete answer:
(i) Propanone to propan−2−ol
Conversion of Propanone to propan−2−ol is done by reduction. Propanone is a carbonyl compound with functional groups.
We have to convert propanone with CO functional group to alcohol with −OH functional group. When we reduce carbonyl compounds in the presence of a reducing agent, they get converted to alcohol. So we will treat propanone with a reducing agent like lithium aluminum hydride (LiAlH4) and Sodium borohydride (NaBH4). On treatment with (LiAlH4) propanone will be reduced to propan−2−ol . It is also called isopropyl alcohol. The reaction will be:
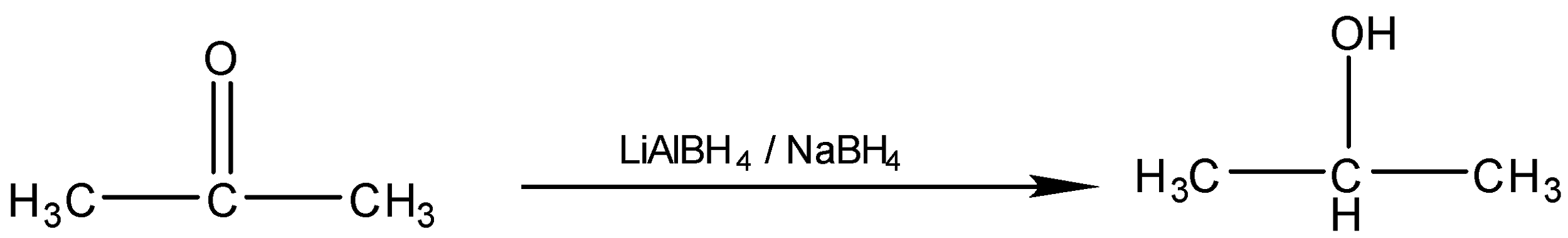
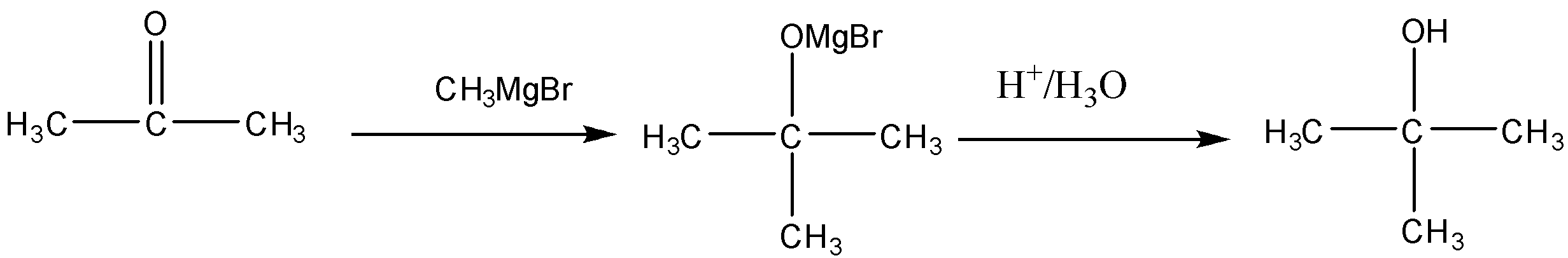
(ii) Propanone to 2−methylpropan−2−ol
The conversion of Propanone to 2−methylpropan−2−olis a two-step process. So First we will treat the Ketone with Grignard reagent i.e. R−MgX where R is the methyl group and X is the halogen. After that, we have to do the acidic hydrolysis of the compound. When we react Propanone with CH3−MgBr, the reagent will break into two parts CH3− and MgBr+, so oxygen being the electronegative atom will attract the shared electron pairs towards itself creating polarity in the bond. The CH3− electrophile will attack there and forms the bond with the carbonyl carbon and MgBr+ will bond with oxygen forming an intermediate. In the second step, the acidic hydrolysis of the intermediate is done to replace the O−MgBr group with −OH. The reaction of the conversion will be:
Note:
propan−2−ol or isopropyl alcohol is a colorless, flammable organic compound that has a very strong odor. It is used in the manufacturing of antiseptics, disinfectants, and a wide variety of household chemicals.
