Question
Question: How is propane nitrile converted into the propanal to write the equation....
How is propane nitrile converted into the propanal to write the equation.
Solution
The propane nitrile is cyanide. The alkyl cyanides or nitriles can be reduced in presence of metal chloride in dry ether. The nitriles are reduced, the metal chloride provides an electron to the nitrile such that the reaction proceeds through the aldimine intermediate. The hydrolysis of aldimine intermediate results in the aldehyde.
Complete step by step answer:
An alkyl or the aryl cyanides or the alkyl or aryl nitriles when dissolved in ether is reduced with the stannous chloride SnCl2 and hydrochloric acid HCl and gives the aliphatic or aromatic aldehyde.
The reactions proceed through the formation of aldimine hydrochloride which is an unstable compound and easily hydrolysed into the aldehyde.
This reaction of alkyl cyanides (or nitriles) when reduced with stannous chloride and hydrochloric acid in absolute ether followed by hydrolysis to give aldehyde. This reaction is known as Stephen reduction.
The general reaction of the reduction of alkyl cyanide is as shown below:
R-C≡NSnCl2 , HClDry ether ECHO + NH3
The mechanistic approach for the reduction of alkyl nitrile or alkyl cyanides is as shown below:
Step 1) The hydrogen chloride HCl is added to the nitrile, it reacts with the nitrogen of the nitrile and forms the corresponding salt.
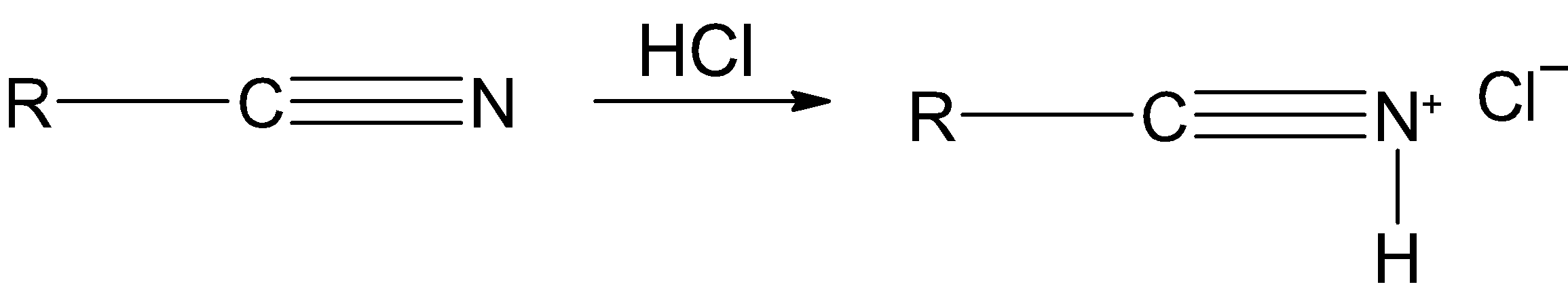
Step 2) The tin (II) chloride SnCl2 is a reducing agent. It transfers an electron to the salt and reduces it as shown below:
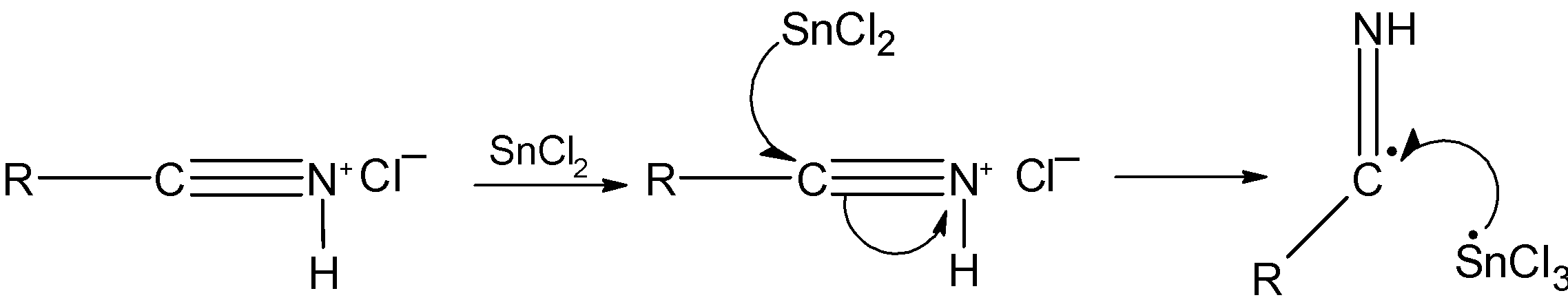
The above step takes place again so that the carbon acquires the electro from the reducing agent and then abstracts a proton from the acid.
Step 3) In the next step, the salt obtained in the above step is precipitated as aldimine tin chloride as shown below:
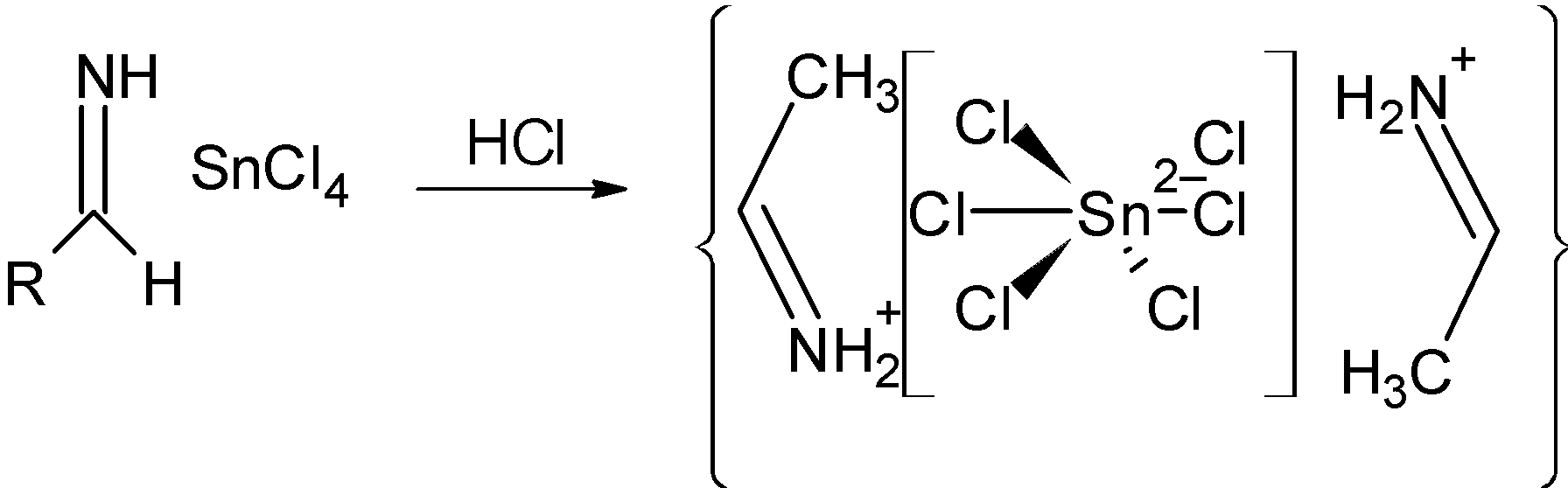
Step 4) The hydrolysis of the above-formed aldimine tin chloride as an amide. In the following step, the amine group is removed NH4Cl and assist the formation of aldehyde. The reaction is as shown below:
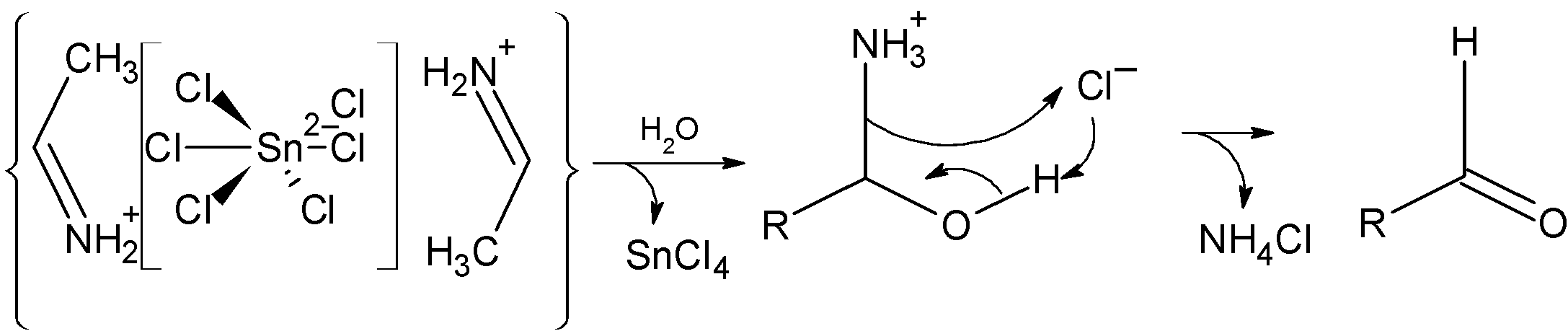
Now let's consider an example asked in the problem. The propane nitrile is a cyanide compound. When treated with the stannous chloride and hydrochloric acid in the dry ether it is reduced as follows:
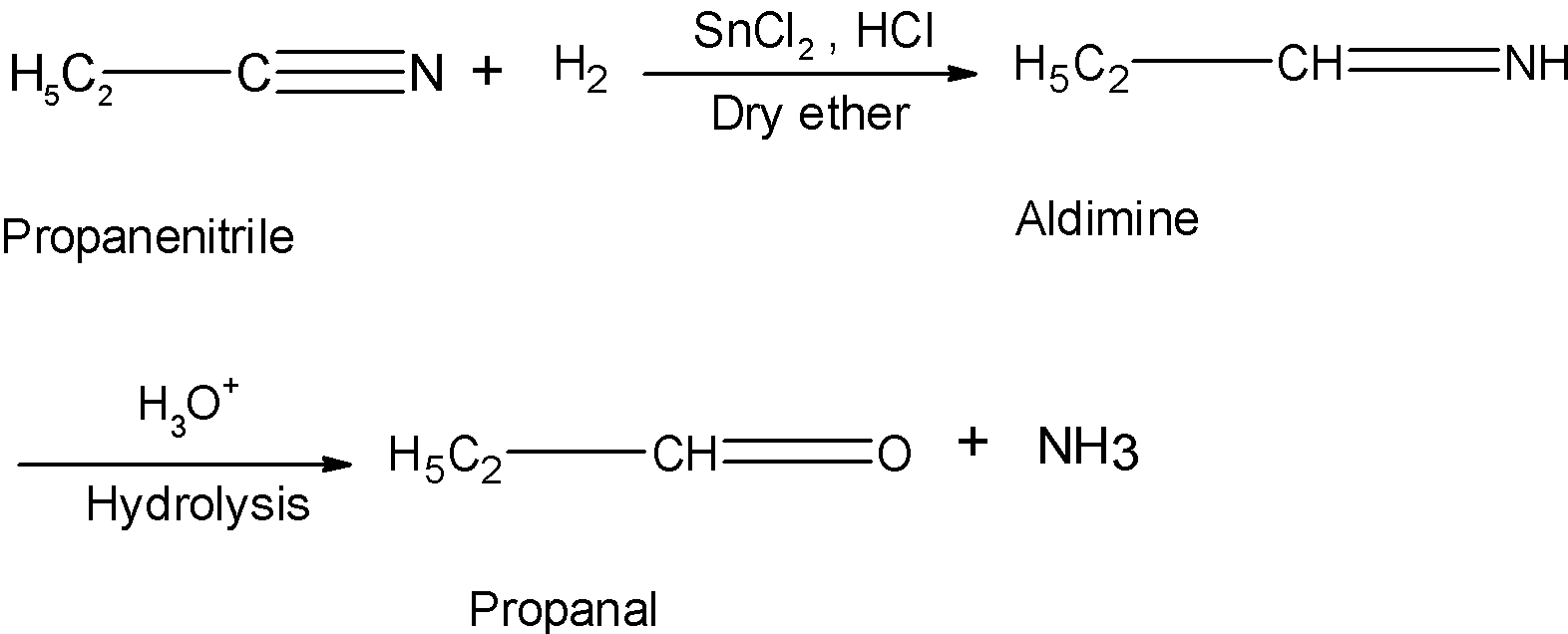
Hence, Stephen's reduction reaction converts the propane nitrile to the propane.
Note: There is an alternate method for the conversion of nitriles to the aldehyde. The nitriles and esters can be reduced with the mild reducing agents such as di-isobutyl aluminium hydride (DIBAL) to imine which on hydrolysis gives aldehyde. The general reaction is as shown below,
RCN1) DIBAL2) H2O , −780CR−CHO
The ketones cannot be prepared by the Stephen reduction method. The bulkier groups' results in obstructing the formation of the product.
