Question
Question: How is nitromethane prepared from (i) \(\alpha - \)chloro sodium acetate (ii) \(\alpha - \)nitro...
How is nitromethane prepared from
(i) α−chloro sodium acetate
(ii) α−nitro isobutylene?
Solution
Nitroalkanes can be obtained by treating alkyl halides with silver nitrate in alcoholic solution. Hydrolysis of α−nitro alkene can produce nitromethane.
Complete step by step solution:
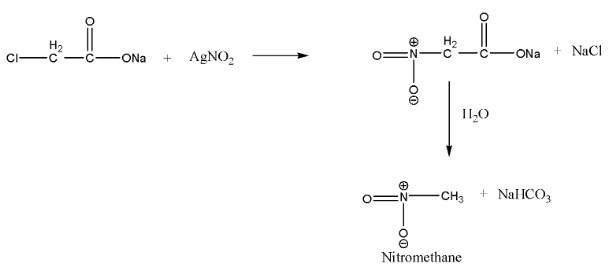
When α− chloro sodium acetate is treated with silver nitrate, the chlorine gets replaced by the nitro group and α−nitro sodium acetate is formed, which undergoes hydrolysis to give nitro methane and sodium bicarbonate as a by-product.
This reaction is a nucleophilic substitution reaction. Since silver nitrite is predominantly covalent, only nitrogen pairs are available for bond formation. Hence, on adding AgNO2, the attack of NO2− takes place mainly through nitrogen and a nitroalkane is obtained as the major product.
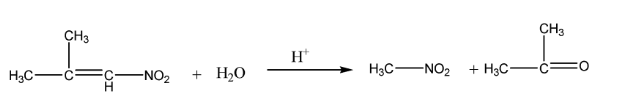
α−nitro isobutylene undergoes hydrolysis in an acidic medium to give nitromethane and acetone.
Note: When α− chloro sodium acetate is treated with silver nitrate, some alkyl nitrate is also formed (20−30%) in the reaction along with nitroalkane. This is becauseNO2− ion is an ambident nucleophile and can attack the alkyl halide through nitrogen as well as through oxygen. If α− chloro sodium acetate is treated with NaNO2 or KNO2 in place of AgNO2, the main product will be alkyl nitrite. Nitroalkenes do not undergo hydrolysis in the basic medium to form nitromethane.
