Question
Question: How is hydrogen bonding among water molecules related to the structure of the water molecule?...
How is hydrogen bonding among water molecules related to the structure of the water molecule?
Solution
We know that hydrogen bonding is one of the strongest molecular forces (second only to ionic bonding). Hydrogen bonding does influence the water molecules when they are in bulk or in solution as they have strong interaction between themselves.
Complete step-by-step answer: Let’s understand how the structure of water molecules plays a role in hydrogen bonding.
Also, oxygen and hydrogen atoms are connected by a covalent bond where a shared pair of electrons is pulled by an oxygen atom and becomes partially negative-charged. So, ultimately, the H atom will become partially positive-charged. When we talk about the bulk of water molecules, then there occurs an interaction between the positive charge of H atom of one molecule and negative charge of O atom of the neighboring molecule. This interaction is very strong and hence named hydrogen bonding.
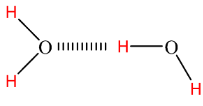
Hydrogen bonding is the reason behind adhesion and cohesion property of water as well as its surface tension.
Generally hydrogen bonding would not occur if there is only a single molecule of water. This hydrogen bonding leads to some unusual but important properties. It helps to give distinctive properties to water and ice (as ice (H2O) has lower density than liquid (H2O)). Due to strong hydrogen bonds, water molecules are able to stay condensed in liquid state.
Note: Remember that Hydrogen bonding can occur in those molecules where hydrogen atom is covalently bonded to highly electronegative atoms such as Fluorine, Oxygen or Nitrogen because they tend to withdraw electron density of the covalent bond with Hydrogen atom, making itself completely electron deficient. Hence H atoms tend to act like a proton which gets attracted towards lone pairs easily.
