Question
Question: How is ethylene glycol made?...
How is ethylene glycol made?
Solution
Catalytic oxidation of alkenes with silver oxide produces compounds that contain a hydroxyl group on their neighboring or adjacent atoms are known as diols. Ethylene glycol is a type of a diol.
Complete answer:
Ethylene glycol has the IUPAC name of ethane-1,2-diol. It is a type of glycol, organic compounds that have neighboring hydroxyl groups. Ethylene glycol has an empirical formula C2H6O2.
Ethylene glycol can be made from the reaction of ethylene when exposed to catalytic oxidation in the presence of an oxidizing agent, produces ethylene oxide, which on hydrolysis in the presence of a dilute acid or a base, produces ethane-1,2-diol or ethylene glycol. The reaction is as follows:
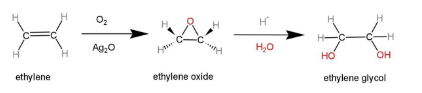
Silver oxide is used as an oxidizing agent here, which forms a tricyclic state of ethylene oxide, that is treated with a dilute acid.
Hence, ethylene glycol is prepared by the above reactions.
Additional information:
Ethylene glycol is of great importance as it is used as an anti-freezing agent for vehicle engines; in air conditioner; it is used in solvents of paints, shoe polish, printing inks; also it is used as a raw material for making various polymers (like polyester). Other uses include in explosives and resins, also in synthetic wax. It is considered as highly poisonous and its consumption by humans or animals can cause fetal illnesses or even death.
Note: Apart from oxidation of ethylene, ethylene glycol can also be prepared from oxalic esters. Oxalic esters, when reduced in presence of alcohol and sodium will form ethylene glycol as a product.
