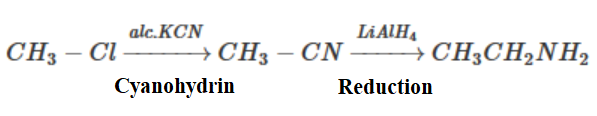Question
Question: How is ethylamine prepared from methyl iodide?...
How is ethylamine prepared from methyl iodide?
Solution
To answer this question, you should recall the concept preparation of amines from haloalkanes. A primary amine is formed when alkyl halide reacts with ammonia. This can be used further to produce other substituted amines.
Complete Step by step solution:
Ethylamine is a colourless/light yellow liquid with a strong ammonia-like odour and is used in the preparation of various pharmaceutical products. It condenses just below the room temperature to a liquid miscible with mostly all solvents. It acts as a nucleophilic base. Let us discuss the easiest procedure to form ethylamine from methyl iodide
1.Methyl iodide is treated with NaCN to form acetonitrile
CH3−I+NaCN→CH3−CN+NaI
This is an example of SN2 a reaction
-SN2 reactions are bimolecular reactions in which there are simultaneous bond-making and bond-breaking steps.
-SN2 reactions do not proceed via an intermediate.
-SN2 reactions result in inverted stereochemistry at the reaction centre.
-Steric effects are particularly important in SN2reactions.
2.Acetonitrile is reduced with Na/ ethanol to form ethylamine. This reaction is called Mendius reduction
CH3−CN→CH3−CH2−NH2(in presence of Na/Ethanol)
Additional Note: Ethylamine can also be synthesised from the reaction of ethane and ammonia in the presence of a basic catalyst. Ethylamine is a weak base and undergoes reactions like acetylation and protonation. It can also be oxidised to form acetaldehyde.
Note: Another way of preparing ethyl amine prepared from methyl iodide is by using cyanohydrin reduction. Methyl iodide first is converted into methyl chloride using the ion-exchange method: CH3I + NaCl →CH3Cl+ NaI.
Now the second reaction can be performed: