Question
Question: How is copper extracted from its sulfide ore? Explain the various steps supported by the chemical eq...
How is copper extracted from its sulfide ore? Explain the various steps supported by the chemical equation. Draw labeled diagram of the electrolytic refining of copper.
Solution
Copper sulfide is oxidized with a metal that is more reactive than the copper element. Smelting is also involved in this process. Arsenic and antimony are the impurities that are present during the refining.
Complete answer:
The sulfide ore i.e., copper pyrites after the concentration by froth flotation process is roasted in a reverberatory furnace when copper pyrites are converted into a mixture of FeS$$$$C{{u}_{2}}S and which, in turn, are partially oxidized.
2FeCuS2+O2→Cu2S+2FeS+SO2
2FeS+3O2→2FeO+2SO2
2Cu2S+3O2→2Cu2O+2SO2
Since iron is more reactive than the copper, FeS is preferentially oxidized to FeO than Cu2S to Cu2O. If at all any Cu2O is formed, it combines with FeS and is changed back to Cu2S.
Cu2O+FeS→Cu2S+FeO
Thus, the roasted ore mainly contains Cu2S and along with unreacted FeS
The roasted ore is then mixed with silica (flux) and some powdered coke and heated strongly in a blast furnace. This process is called smelting. During smelting combines with silica to form fusible ferrous silicate slag.
FeO+SiO2→FeSiO3
At the temperature of the furnace, the entire mass melts, and two layers of molten mass are formed. The slag being lighter makes the upper layer which can be withdrawn from the slag whole from time to time. The lower molten layer is called copper matte. It chiefly consists of Cu2S and some unchanged FeS.
Recovery of copper from matte:
To obtain pure copper from matte, the molten matte is transferred to a besimmer converter which is a pear-shaped furnace made up of steel and lined inside with silica. It is mounted on a horizontal axel and can be tilted in any position. It is fitted with small pipes called tuyeres through which a blast of hot air and fine sand is admitted.
During the process of bessemerization, any sulfur, arsenic, and antimony still present as an impurity in matte escape as their respective volatile oxides areFeS is oxidized toFeO which combines with silica from FeSiO3 slag.
2FeS+3O2→2FeO+2SO2; FeO+SiO2→FeSiO3(slag)
The slag thus melts and floats on the top of the molten mass and is removed. When the whole of iron has been removed as slag, some of the cuprous sulfides undergo oxidation to form a cuprous oxide which reacts with cuprous sulfide to form copper metal.
2Cu2S+3O2→2Cu2O+2SO2; 2Cu2O+Cu2S→6Cu+SO2
The diagram of electrolytic refining is given below:
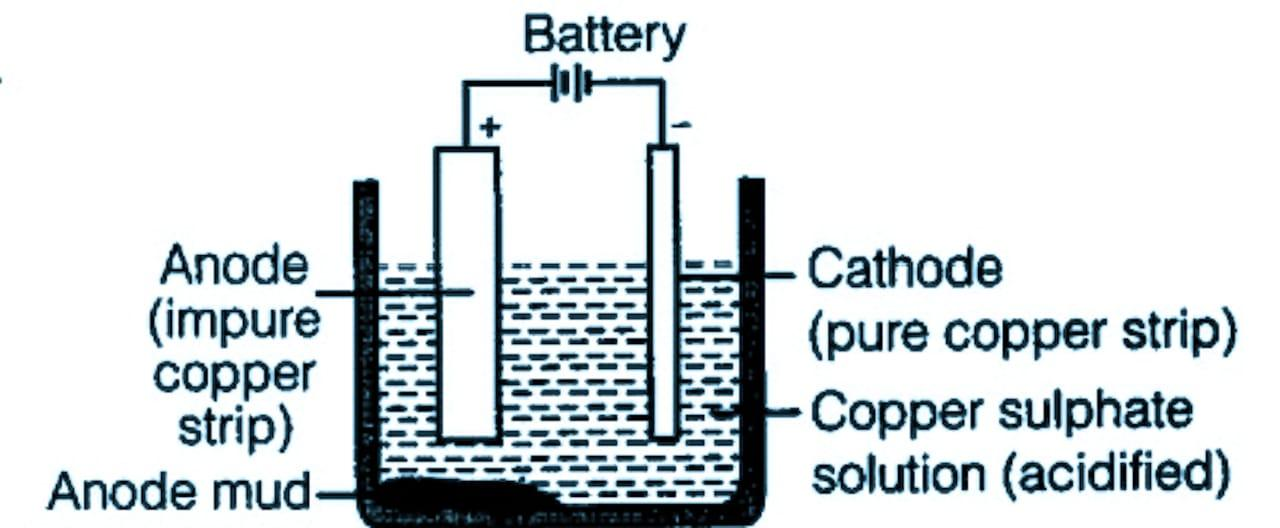
Note: There are two processes of converting copper sulfide to copper. The sulfide is converted to oxide then the oxide is converted into pure copper using coke as the reducing agent. The reducing agent is selected by observing the Ellingham diagram.
