Question
Question: How is Benzene converted to Salicylic acid?...
How is Benzene converted to Salicylic acid?
Solution
Salicylic acid is 2 - Hydroxybenzoic acid. Therefore it contains a OHgroup and a COOHgroup. We have to substitute these two groups on the given benzene. For this we will convert benzene into phenol and will add carboxylic acid .
Complete answer:
Benzene can be converted into Salicylic acid using the following steps :
Step 1.We will first convert Benzene to phenol. There are many through which it can be converted into phenol. One of them is mentioned below:
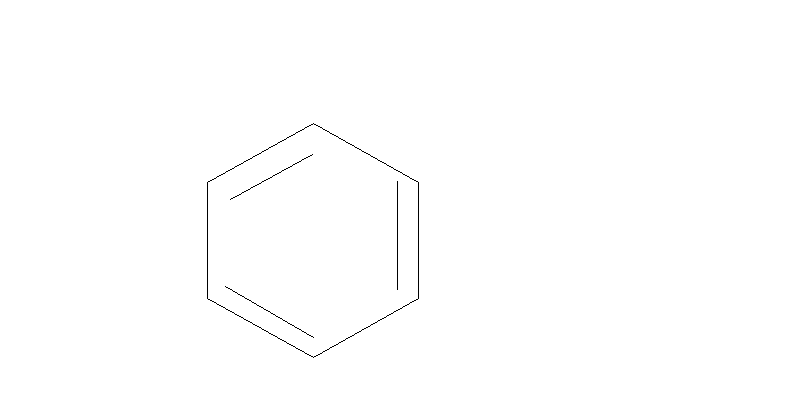 H2SO4
H2SO4 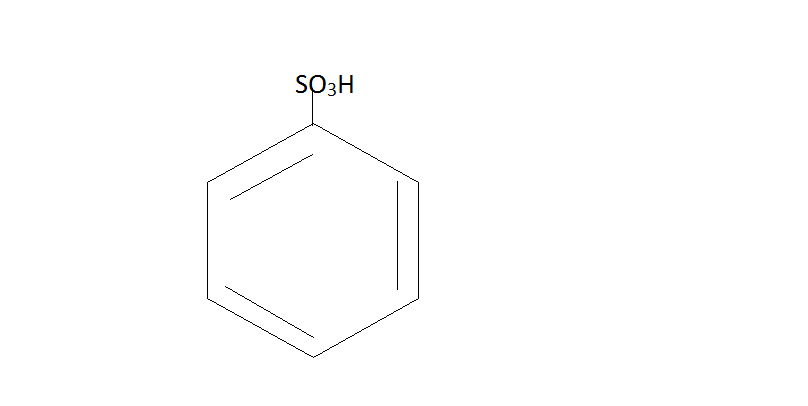 NaOH
NaOH 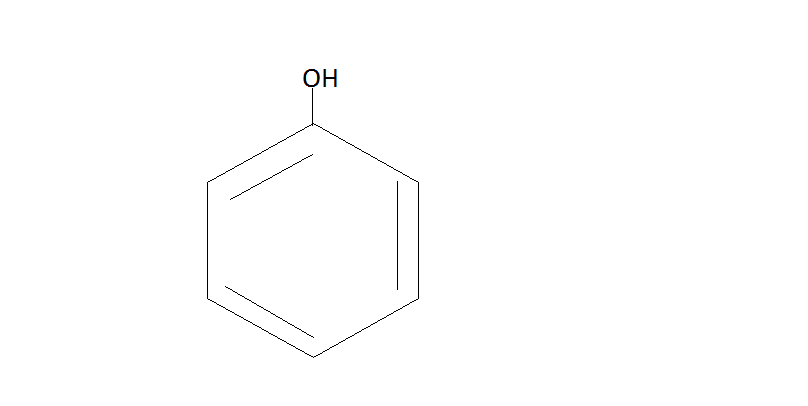
Benzene Benzene Sulfonic acid Phenol
Here Benzene reacts with sulfuric acid to form Benzene Sulfonic acid. Then this acid further reacts with NaOHat 200∘C . This will give us phenol.
Step 2. Now the phenol will react with CO2 / NaOHand then we have to do protonation with help of H2SO4. The phenol reacts with CO2 / NaOH under pressure which will give us sodium salt or salicylic acid. Then the protonation of this salt yields us salicylic acid.
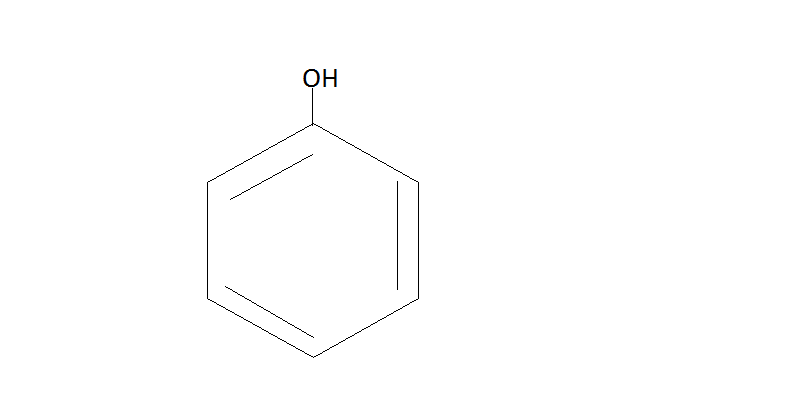 CO2 / NaOH
CO2 / NaOH 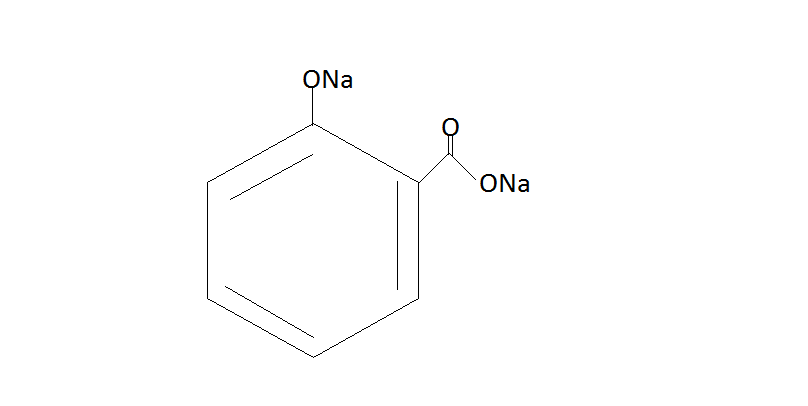
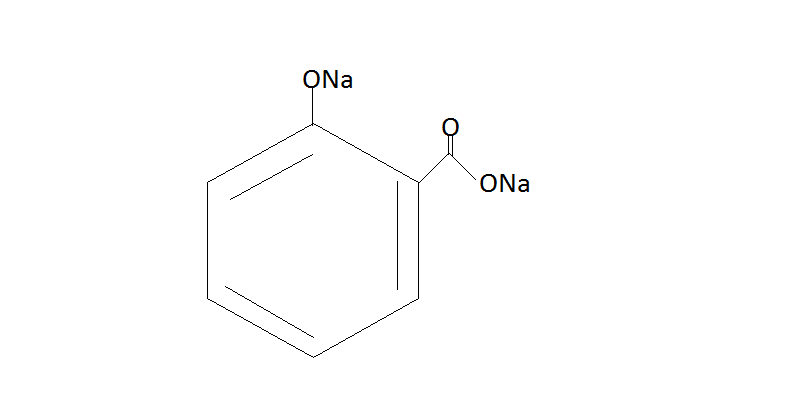 $\xrightarrow{{{H_2}S{O_4}}}$ 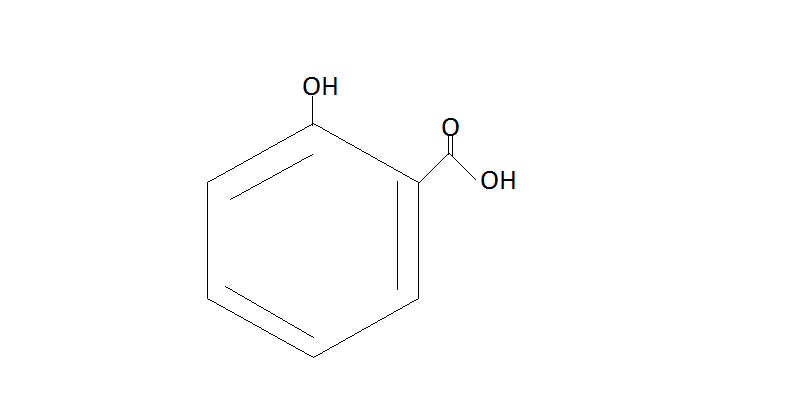
Salicylic acid
Thus we get Salicylic acid from Benzene by using these steps. This reaction is also known as the Kolbe Schmitt Reaction. It is also known as beta hydroxy acid.
Note:
The reaction takes place at high pressure because of the stability of benzene. For protonation we use H2SO4 which gives H+. It is preferable to get the hydroxyl group first on the ring because if the carboxyl group is attached first then the ring becomes meta directing in nature. Hence salicylic acid cannot be formed in this way. Thus the hydroxyl group is added first since it is o - pactivating group. Thus we get salicylic acid as our product.
