Question
Question: How is benzamide converted into benzylamine?...
How is benzamide converted into benzylamine?
Solution
Benzamide can be converted into benzylamine by using LiAlH4 or we can also use NaOH+Br2.
Complete step by step answer:
The most common method of converting benzamide to benzylamine is by LiAlH4 but it requires greater selection because LiAlH4 can also react with others. If there are no other groups that can be affected by LiAlH4, in that case, LiAlH4 is probably the reagent of choice. The more LiAlH4 is required for reduction of amides to amines because the carbonyl carbon of amides is less electrophile, as the electron density is shared by resonance.
The step by step conversion of Benzyl amine into benzamide is as follows:
(i) The LiAlH4 is a strong reducing agent and is used to reduce the carbonyl group into corresponding saturated systems by the addition of hydrogen atoms and removal of oxygen atoms in the form of water.
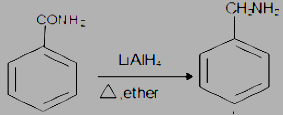
(ii) Heat the compound in the presence of dry ether.
We can also convert it by adding Br2 and NaOH/KOH to benzamide and heat it which will convert it to amine with one carbon less than the reactant, this is called Hoffman Bromamide Degradation Reaction. Then treat it with HNO2 followed by HCN/CuCN to convert it into Benzene cyanate then treat it with H2/Pd. By this way benzamide will convert into benzylamine.
Note:
Red phosphorus + HI can also reduce amides into amines. So, we can also use other reducing agents that can convert amides into amines.Catalytic hydrogenation can be used to reduce amides to amines; however, the process often requires high hydrogenation pressures and reaction temperatures to be effective
