Question
Question: How is ammonia manufactured industrially?...
How is ammonia manufactured industrially?
Solution
Hint:Ammonia is a compound composed of one nitrogen atom and three hydrogen atoms held by covalent bonds. It has a lone pair of the electron over the nitrogen atom.
Complete step-by-step answer:
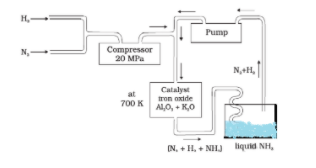
Haber’s process is used for the synthesis of ammonia which is carried out at a high temperature because, at low temperatures, the reaction becomes very slow due to which the rate of formation of ammonia becomes very slow.
To make ammonia, the mixture of N2 and H2 gases are passed over heated iron. Heated iron acts as a catalyst in the synthesis of ammonia. It accelerates the rate of reaction.\
The following reaction will take place:
N2(g)+3H2(g)⇌2NH3
A mixture of dry nitrogen and hydrogen in the ratio of 1:3 by volume is compressed in the presence of pressure approx. 200−300atm and passed over the Fe catalyst at a temperature of approximately 723−773K.
Now, the Fe catalyst mixed with aluminium oxide and potassium oxide which acts as promoters.
The ammonia formed is continuously removed by liquefaction.
Additional Information:
Le chatelier's principle is the principle that determines how the equilibrium of a reaction changes by changing various factors on which it depends. According to this principle, change in any of the factors that determine the equilibrium conditions of a system will cause the system to change in such a manner so as to reduce or counteract the effect of the change.
These factors include the concentration of reactant, product, temperature, and catalyst.
Note: The possibility to make a mistake is that you may forget that this formation of ammonia by Haber’s process occurs in the presence of a catalyst that acts as a promoter.
