Question
Question: How does the concentration of an alcohol - water solution affect the volume contraction?...
How does the concentration of an alcohol - water solution affect the volume contraction?
Solution
Volume compression is one of the signs of DKA. It can add to acidosis through lactic corrosive creation and diminished renal freedom of natural and inorganic acids.
The measure of volume compression or extension is noticeable in charts of absolute fume pressing factor and halfway fume pressures comparative with ideality.
Complete step by step answer:
The measure of volume compression or extension is obvious in charts of absolute fume pressing factor and incomplete fume pressures comparative with ideality
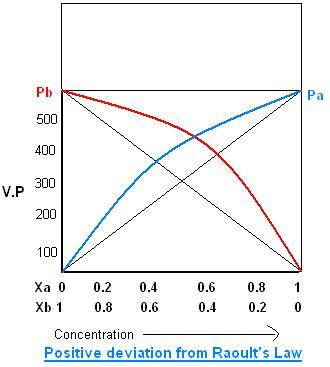
P∘ is the fume pressing factor of the unadulterated substance, and PR is the fractional pressing factor of R while blended in with S. The top bend is the absolute fume pressure.
The best volume deviations happen at a 50:50 by mols combination. The course of volume deviation is equivalent to the bearing of progress in absolute fume pressure.
When there is no volume change in the wake of blending, the complete fume pressure is consistent, as per Raoult's law for ideal arrangements.
When the volume contracts in the wake of blending, the complete fume pressure is more modest than anticipated (in light of the fact that the atoms interface all the more unequivocally, and less fluid at the surface can disintegrate).
When the volume extends in the wake of blending, the absolute fume pressure is bigger than anticipated (in light of the fact that the particles connect not so much unequivocally, but rather more fluid at the surface can disintegrate).
This is by and large. The impacts of fixation (as far as mol portions) are appeared beneath in more detail.
WHY VOLUME DEVIATIONS FROM IDEALITY OCCUR
The more comparable the intermolecular powers (IMFs) of the solute and dissolvable, the more effectively they blend ("like breaks up like").
At the point when more grounded IMFs structure among solute and dissolvable than they each initially had, the volume agreements, and the other way around.
Pattern WITH CONCENTRATION (MOL FRACTION SCALE)
For the charts beneath:
χ1 demonstrates the mol division of CS2(CHCl3) in the arrangement stage in the principal (second) diagram.
χ2=1−χ1 shows the mol part of H3COCH2OCH3(H3C(C=O)CH3) in the arrangement stage in the main (second) chart.
Note: Nonetheless, for methanol and ethanol, whose alkyl chains are short (delivering them reasonably hydrophilic),When all is said in done, it is seen that the best impact on fume pressing factor (and in this manner volume deviations) happen nearest to a 50:50 combination as far as moles.
