Question
Question: How does pH affect amino acid structure?...
How does pH affect amino acid structure?
Solution
We need to know the amino acid structure and accordingly understand the effect of pH on its structure. An amino acid is an organic molecule that is made up of a basic amino group (−NH2) , an acidic carboxyl group (−COOH) , and an organic R group (or side chain) which is unique to each of the 20 amino acids, and a hydrogen atom. In addition to the structure, we must know that the amino acids are amphoteric in nature, that is they can act both as an acid or a base hence they are greatly affected by pH.
Complete step by step answer:
We need to know that since amino acids are amphoteric in nature, they can act both as an acid or a base hence affected by pH. They have the capacity to protonate as well as deprotonate. Here the concept of pKa comes into picture. The pKa value given for the amino group on any amino acid specifically refers to the equilibrium between the protonated positive nitrogen and deprotonated neutral nitrogen. Both the amino as well as the carboxyl groups have the capacity to protonate as well as deprotonate, hence it has two pKa values.
-The pKa of the carboxyl group is always lower than that of the amino group hence when pH increases, the carboxyl group will be deprotonated before the amino group.
-The pKb values for amino groups are lower than that of carboxyl groups, hence the amino groups will be protonated before the carboxyl groups.
The structures of amino acids with respect to pH is given below:
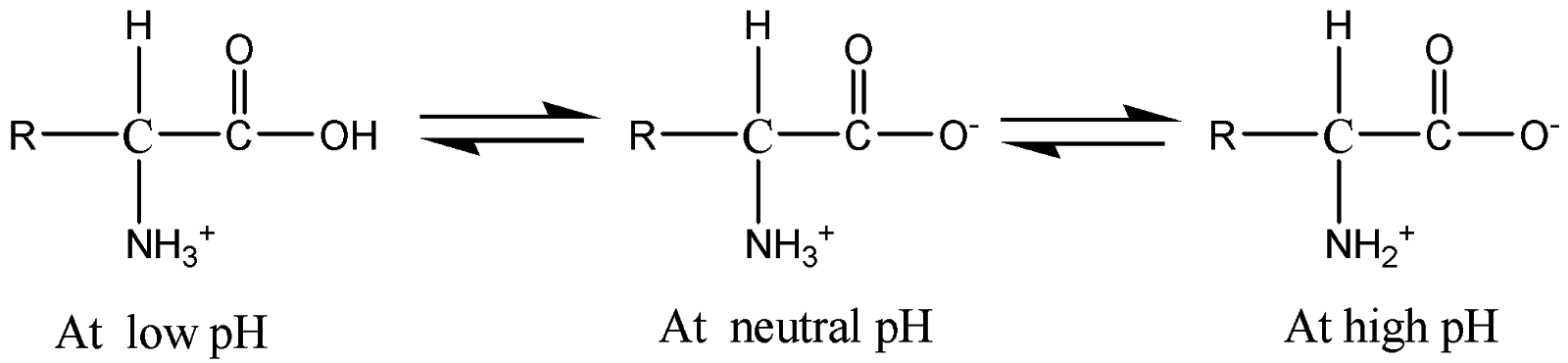
The forward arrow represents deprotonation and the backward arrow represents protonation of amino acids with respect to pH.
Hence pH directly affects the structure of amino acids as a slight increase in pH will protonate and deprotonated the amino acid.
Note:
It must be noted that amino acids are zwitterionic in nature. A zwitterion is a compound that has no overall charge but that has charge separation within it. An amino acid is therefore zwitterionic at neutral pH and this pH is known as the isoelectric point. The zwitterionic nature of amino acids has an effect on their properties.
