Question
Question: How does ionic bonding relate to the Lewis Theory?...
How does ionic bonding relate to the Lewis Theory?
Solution
The diagrammatic representation of the electron distribution around the atoms in a molecule is known as a Lewis dot structure. To draw the Lewis dot structures we must know the valence electrons on each atom present in the given ionic compounds. Then calculate the number of total valence electrons of the ionic compounds.
Complete answer:
We know that the diagrammatic representation of the electron distribution around the atoms in a molecule is known as a Lewis dot structure. The steps to draw the Lewis dot structure of ionic compounds are as follows:
Determine the total number of valence electrons.
Select the central atom.
Draw the skeletal structure.
Determine the number of bonds in a molecule.
Determine the number of electrons required for an atom to complete its octet.
Place the electrons on the outside atoms.
Place the remaining electrons around the central atom.
For example consider an ionic compound magnesium chloride. The valence electrons of magnesium are two and the valence electrons of chlorine are 7. Thus, Total valence electrons of MgCl2=2+(2×7)=2+14=16
The central atom is magnesium. The two chlorine atoms are around the magnesium atom. The skeletal structure is as follows: Cl−Mg−Cl. Here, the number of bonds is two. Thus, four electrons are involved in bonding. Thus, remaining electrons are, 16−4=12.
Now, each chlorine atom requires six electrons to complete its octet. Thus, place six electrons around each chlorine atom. Thus, the Lewis dot structure of is as follows:
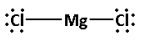
Note:
Lewis dot structures are also known as electron dot structures. To draw the Lewis dot structures remember the atomic numbers of the elements in the compounds. This helps in easily determining the valence electrons and thus helps in determining the Lewis dot structure.
